High Entropy Oxides
Stable solid-solution phases consisting of five or more primary elements can be achieved due the high entropy of mixing stabilizing the structure, overcoming enthalpy minimization driven segregation. This has initially been realized in high entropy alloys (HEA), which exhibit unique mechanical properties superior to the traditional alloys. Research work on high entropy materials has been extended to oxide systems with rock salt MO, spinel M3O4 or perovskite ABO3 structures where ‘M’, ‘A’ and ‘B’ represent five or more nearly equiatomic metal cations. The high configurational entropy can effectively stabilize single phases of such complex multicomponent oxide systems, giving rise to new functional properties. Recently, the concept was extended to multi anionic high entropy materials such as LiMOF, which could be synthesized successfully. The atomic and electronic structure of HEAs and HEOs is of great interest and depends on the choice of substitutional elements. The geometric structure and electronic state of the cations will in turn determine the mechanical, electric and magnetic properties of the materials. We are investigating the atomic and electronic structure of entropy-stabilized materials with state-of-the-art transmission electron microscopy.
A detailed TEM study was carried out on the 10 cation system (Gd0.2La0.2Nd0.2Sm0.2Y0.2)(Co0.2Cr0.2Fe0.2Mn0.2Ni0.2)O3, providing direct proof of the local homogeneity and the phase purity. Figures 1a-c show the HAADF STEM images along [100], [121] and [011] zone axis. STEM-EDX maps with atomic resolution from the [011] zone axis are shown in Figure 2. The distributions of A and B cations on each position are homogeneous. The EDX signals from site A and site B were summed up and are shown in the color mix map in Fig. 2c (green for site A and red for site B). In the HAADF STEM image in Fig. 2a, the heavier A site atoms can be readily discerned as zigzag chain along [1 ̅01] direction, while the B site atoms form a regular lattices. iDPC images obtained using a segmented STEM dark field detector yields contrast proportional to the projected potential of the crystal, enabling detection of light elements in addition to the heavy elements. A iDPC STEM image acquired from the same area as Fig. 1c and the STEM-EDX mapping is shown in Figure 1d. The heavier rare earth cations are clearly distinguishable from the transition metal cations.
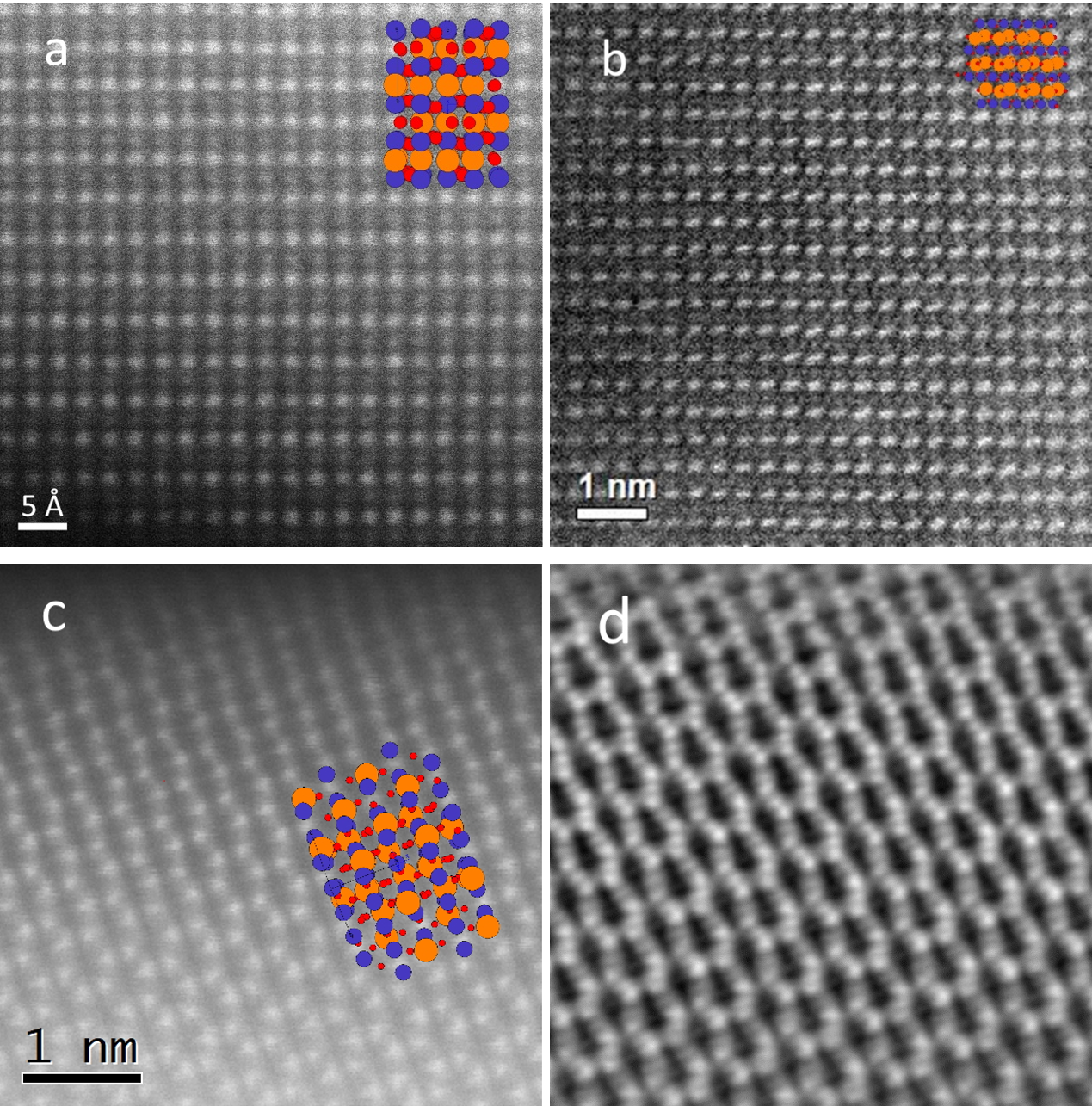
Fig. 1a-c HAADF STEm images of (Gd0.2La0.2Nd0.2Sm0.2Y0.2)(Co0.2Cr0.2Fe0.2Mn0.2Ni0.2)O3 on
the [100], [121] and [011] zone axis; d the iDPC image from the same area of Fig. 1c.
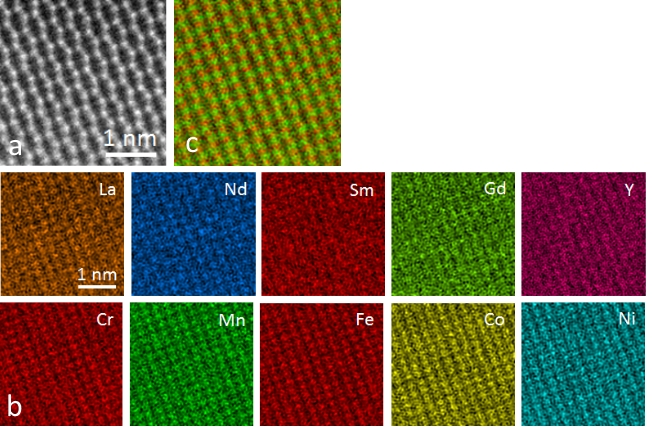
Fig. 2 STEM-EDX maps taken along [011] zone axis, where the atomic columns containing heavier atoms (Gd, La, Nd, Sm and Y) and lighter atoms (Co, Cr, Fe, Mn and Ni), respectively can be clearly distinguished.
Details of sample preparation and primary structure characterization can be found in at:
- Rare earth and transition metal based entropy stabilized perovskite type oxides.
Abhishek Sarkar, Ruzica Djenadic, Di Wang, Christina Hein, Ralf Kautenburger, Oliver Clemens, and Horst Hahn.
Journal of the European Ceramic Society, 2018, 38(5), 2318–2327. doi:10.1016/j.jeurceramsoc.2017.12.058.
HEO materials with rock-salt structure containing transition metals have been successfully synthesized and applied as active materials in reversible energy storage. In recent work, we characterized the as-prepared and cycled HEO samples used in lithium ion batteries (Fig. 1) and reported the underlying mechanisms governing the excellent reversibility. After cycling, a pseudo long-range “ordered” structure, containing many defects, is observed (Fig. 1b). The lattice spacings, corresponding to the (111) and (200) planes of the rock-salt structure, are still clearly visible in the high-resolution image (Fig. 1b) and in its fast Fourier transform (FFT) (Fig. 1c). Figure 1d shows STEM-EDX maps of the TM-HEO after the initial cycle. EDX of the cycled material does not show any qualitative differences in elemental distribution compared to the as-prepared TM-HEO.
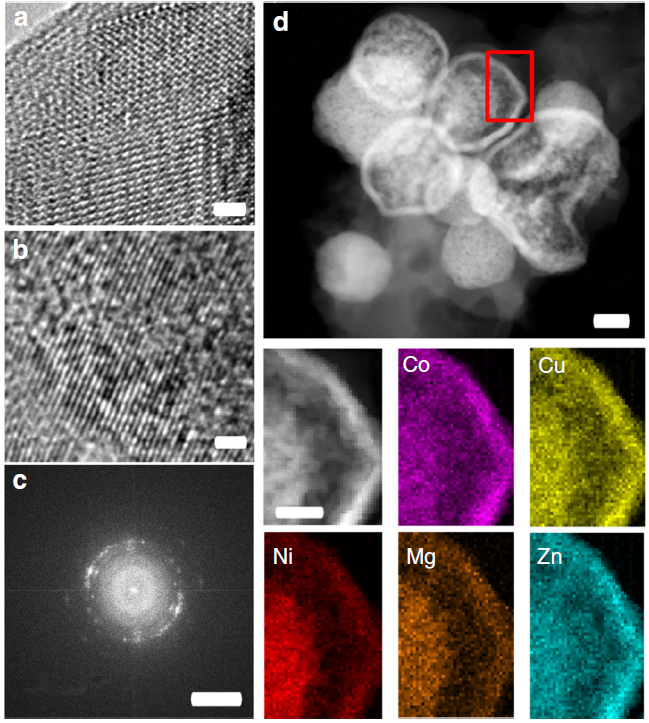
Fig. 1 HRTEM and EDX analysis of the active TM-HEO: a, b HRTEM images of the as-prepared and cycled TM-HEO. The crystallites in b are substantially smaller and do not show long range order. The lattice fringes in b are not completely straight, but show small viations from the original axis. Nevertheless, the small regions with dimensions on the order of a few nanometers exhibit lattice fringes oriented in a specific direction, likely due to the former long-range order, which is partially lost during the conversion reaction. c FFT of a HRTEM image of a few crystallites. The arcs in the FFT correspond to the (textured) cubic rock-salt structure. The lattice fringes are discontinuous between different crystallites; the misorientation is small, caused probably by large amounts of defects or low angle boundaries. d STEM image showing the spheres consisting of small particles with the reference to the elemental maps (red rectangle). No apparent segregation is noticeable at the length scale of aggregates of nanoparticles. The scale bars in a and b correspond to 1 nm, in c to 5 1/ nm, in d to 200 nm and for the compared EDX analysis to 60 nm.
More details can be found in:
- High entropy oxides for reversible energy storage
Abhishek Sarkar, Leonardo Velasco, Di Wang, Qingsong Wang, Gopichand Talasila, Lea de Biasi, Christian Kübel, Torsten Brezesinski, Subramshu S. Bhattacharya, Horst Hahn and Ben Breitung
Nature Communications, 2018, 3400; doi:10.1038/s41467-018-05774-5.
Based on the HEOs, a new class of high entropy materials for energy storage applications has been introduced. Multi-anionic and -cationic compounds are prepared by a facile mechano-chemical synthesis using a recently designed multi-cationic transition-metal-based high entropy oxide as the precursor and LiF or NaCl as the reactant, leading to formation of lithiated or sodiated materials. TEM and HRTEM have been used to characterize the structure of this entropy-stabilized material. EFTEM was carried out revealing a uniform elemental distribution (Fig. 1c). Fig. 1 shows results from HRTEM and SAED on the Li(HEO)F. The aggregates have an average size of 100 nm (Fig. 1a) with a crystalline rock-salt structure (Fig. 1b), while the SAED pattern shown as inset reveals no other (crystalline) secondary phases.
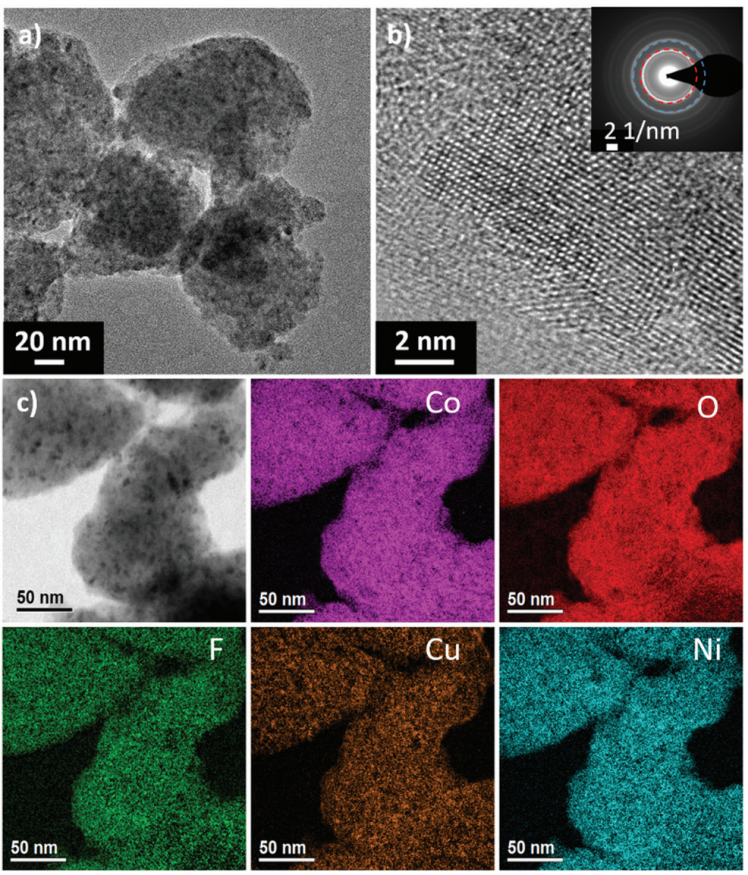
Fig. 1 (a and b) TEM images at different magnification of the as-prepared Li(HEO)F showing crystalline particles around 100 nm in size. The inset in (b) is an SAED pattern obtained on the same material. The (200) and (220) reflections are denoted by red and blue circles, respectively. (c) EFTEM mapping results revealing a uniform element distribution.
More details can be found in:
- Multi-anionic and -cationic compounds: new high entropy materials for advanced Li-ion batteries.Qingsong Wang, Abhishek Sarkar, Di Wang, Leonardo Velasco, Raheleh Azmi, Subramshu S. Bhattachary, Thomas Bergfeldt, Andre Düvel, Paul Heitjans, Torsten Brezesinski, Horst Hahn and Ben Breitung.
Energy Environ. Sci., 2019, 12, 2433-2442. doi: 10.1039/C9EE00368A.

