Molecular Motors and Switches
The capability of light-induced E/Z
isomerization of simple imines (-N=C-), their ubiquity and their ease of
preparation feature them as promising candidates for axle-functionalities in
molecular switches and motors. Nevertheless, this class of compounds was mostly
neglected for such purposes, possibly due to the thermal instability of the
formed photoisomers.
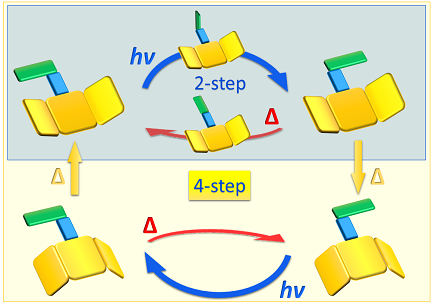
Besides establishing some essential switching characteristics, we demonstrate that chiral N-alkyl imines undergo unidirectional rotation induced by light and heat.1 Depending on the conformational flexibility of the stator part (the carbonyl residue) and the nitrogen inversion barrier of the rotor part (the amine residue) in the molecule, the operation mode of the motor can be considered as either a 4-step or a 2-step cycling motion of the rotor part.

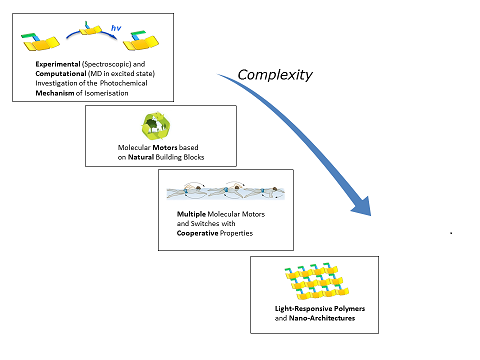
The essential mechanism of the photochemical C=N bond rotation shall be clarified by experimental and computational methods. The benefits of imines as molecular switches with potential extensions in different directions will be investigated. Such efforts include varying levels of complexity: from generation of molecular motors based on readily available natural products, over multiple molecular motors/switches towards large polymeric and nano-structures with the ability to respond to light.
1. L.
Greb, J.-M. Lehn J. Am. Chem. Soc., 2014, 136 ,
13114–13117.