Nano-Objects and Architecture
Nanoscale objects display particular interesting physical properties due to their size restrictions for electronic waves. Quantization effects are ruling their physics giving new challenges and opportunities for both, fundamental research and potential applications. Therefore, the control over size, shape and material properties of nanoscale objects is crucial to design and tailor their physical properties. Nowadays two different strategies are pursued to fabricate nanoscale objects. While improved lithographical and other physical methods allow to reduce the manufactured objects size (top-down approach), advanced synthetic chemistry and supramolecular approaches constantly increase the size of available molecules and super-molecules ("bottom-up approach"). Synthetic chemistry can be understood as engineering at the nanoscale and allows to assemble nanoobjects with very particular physical properties.
1. Self Assembled 2D chiral porous networks
|
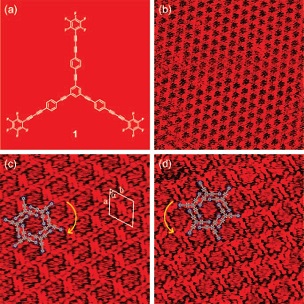
Branched star-shaped molecule 1 and STM image displaying the 2D porous chiral network. |
Self-assembly of the partially fluorinated rigid molecules physisorbed at solution/graphite interface has been investigated by scanning tunneling microscopy. Upon adsorption, the branched star-shaped compound 1 compromising diacetylene and acetylene interlinked benzene and pentafluorobezene formed two-dimensional chiral porous networks. The spontaneous formation of these architectures is likely attributed to the two effects: the compensation of the dipole moments of the branches and the formation of Ar−H···F hydrogen bonds. These results demonstrate that the immobilization of molecules at the liquid/solid interface can be driven by these weak intermolecular interactions instead of van der Waals interactions between alkyl chains and substrate. J. Am. Chem. Soc. 2008, 130, 10840-10841. Link |
2. Shape persistent macrocycles
Shape-persistent macrocycles (SPMs) have attracted considerable attention during the last decade. As rigid nanoscale scaffolds, they are not only able to control the intramolecular spatial arrangement of functional groups and subunits, but also to organize their intermolecular self assembly in solution and on 2D surfaces. Furthermore, their aggregation properties make them promising building blocks for nanoscale objects and tailor-made functional materials.
|
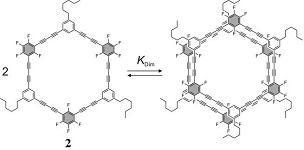
Equilibrium between macrocycle 2 and its dimer complex |
The synthesis and characterization of a shape-persistent macrocycle 2, consisting of alternating electron rich and electron poor sub-units as a self-complementary recognition pattern, is reported, and its increased tendency to form dimer complexes in solution is demonstrated and discussed. Chem. Comm. 2006, 4134-4136. Link Org. Biomol. Chem. 2009, 7, 1081-1091. Link |