Cancer Diagnostics
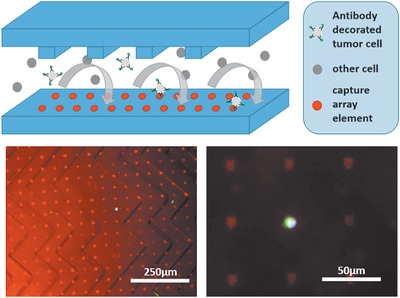
Cancer is a leading cause of death in developed countries and with changing demographics and economic developments, this trend will continue world-wide. Early detection of cancer is connected with longer survival times, better treatment options and higher chance of complete curing. Additionally, current diagnostic methods often rely on biopsies of the primary tumor, which are invasive, painful and potentially dangerous for the patient, depending of type and location of the tumor. An emerging alternative for diagnostic purposes is the "liquid biopsy" that is sampling tumor-associated biomarkers released by a tumor from a simple blood drawing. One attractive target in such a liquid biopsy are cells emitted by the primary tumor into the bloodstream. These circulating tumor cells (CTCs) are the vehicle of metastasis and offer a wider range of diagnostic information. Number of CTCs is correlated with prognosis of disease progression and the cells can be used for genetic analysis of the tumor or even be used for cell culture and xenografts. To detect CTCs out of a huge background of healthy blood cells, highly effective and specific capture methods are needed.
Here, SPL methods can create large area capture arrays that - in combination with anti-fouling surfaces and microfluidc chambers - are able to not only isolate CTCs but make them accesible to further downstream analysis. Together with the medical group of Prof. Klaus Pantel at the UKE in Hamburg, we are developing complete microfluidic chip devices that can translate this approach into regular clinical use.
Selected Publications
A Versatile Microarray Platform for Capturing Rare Cells
F. Brinkmann, M. Hirtz, A. Haller, T. M. Gorges, M. J. Vellekoop, S. Riethdorf, V. Müller, K. Pantel, H. Fuchs
Sci. Rep. 5 (2015) 15342, DOI:10.1038/srep15342
Evaluation of Microfluidic Ceiling Designs for the Capture of Circulating Tumor Cells on a Microarray Platform
H.-Y. Liu, C. Koch, A. Haller, S. A. Joosse, R. Kumar, M. J. Vellekoop, L. J. Horst, L. Keller, A. Babayan, A. V. Failla, J. Jensen, S, Peine, F. Keplinger, H. Fuchs, K. Pantel, M. Hirtz
Adv. Biosyst. 4 (2020) 1900162, DOI:10.1002/adbi.201900162
Biosensing with Cucurbiturils

Together with the group of Dr. Frank Biedermann, we explore the potential of surface bound cucurbiturils (CBs) as biosensors. In general, CBs can act es highly specific non-biological hosts of small biomolecules, like e.g. hormones. When combinined with indicator dyes, these host-guest ineraction can be read-out by e.g. a rise or drop in fluorescence intensity upon analyte binding. This led to the development of indicator-displacement assays (IDA) and guest-displacement assays (GDA) for sensitive and selective detection of analytes. In our project, SPL methods are used to provide patterned and multiplexed arrays of CBs on surfaces that can then be read out via fluorescence microscopy. By bringing the CBs from bulk phase to a surface bound state, we aim to minimize the volume of analyte needed and thus enhancing sensitivity of CB based sensing. Additionally, handling of such arrays will be easier and multiplexed detection becomes feasible.
Selected Publications
Teaching old indicators even more tricks: binding affinity measurements with the guest-displacement assay (GDA)
S. Sinn, J. Krämer, F. Biedermann
Chem. Commun. 56 (2020) 6620-6623, DOI:10.1039/D0CC01841D
Presentation of Allergens
When experimenting with cells and their recognition of the sorrunding, being able to present defined and highly-localized stimuli is often imperative. One example is the study of the interaction between anti-body carrying receptors on immune cells with the matching allergens in order to better understand the recognition event and the subsequent activation of the cell's inner protein machinery as well as the modulation of these by drugs as e.g. steroids. Here, SPL methods can be used to provide large-area patterns of sub-cellular sized spots of model allergens, so interaction between the receptor and allergen occurs at defined and predictable positions making them much easier to observe in clear detail and time-resolved.

Incorporation of these arrays into microfluidic devices allowas for parallel and highly standardized experimentation in several parallel microchannels, furthering statistics and reproducibility of experimental conditions. Having defined and reproducible points of interactions allowed e.g. to pinpoint the locilzation and dynamics of the glucocoticoid receptor in mast cells during activation. Similar approaches can be applied to other cell types and recognition motifs to study adhesion processes or guide cells into certain positions on a sample.
Selected Publications
Allergen Arrays for Antibody Screening and Immune Cell Activation Profiling Generated by Parallel Lipid Dip-Pen Nanolithography
S. Sekula-Neuner, J. Maier, E. Oppong, A. C. B. Cato, M. Hirtz, H. Fuchs
Small 8 (2012) 585-591, DOI:10.1002/smll.201101694
Localization and Dynamics of Glucocorticoid Receptor at the Plasma Membrane of Activated Mast Cells
F. Brinkmann, M. Hirtz, A. Haller, T. M. Gorges, M. J. Vellekoop, S. Riethdorf, V. Müller, K. Pantel, H. Fuchs
Small 10 (2014) 1991-1998, DOI:10.1002/smll.201303677
Click-Chemistry Based Allergen Arrays Generated by Polymer Pen Lithography for Mast Cell Activation Studies
R. Kumar, A. Bonicelli, S. Sekula-Neuner, A. C. B. Cato, M. Hirtz, H. Fuchs
Small 12 (2016) 5330-5338, DOI:10.1002/smll.201601623

