Ion Spectroscopy and Ion Chemistry of Molecules and Clusters

Scientist
Ion Spectroscopy and Ion Chemistry of Molecules and Clusters
Methods
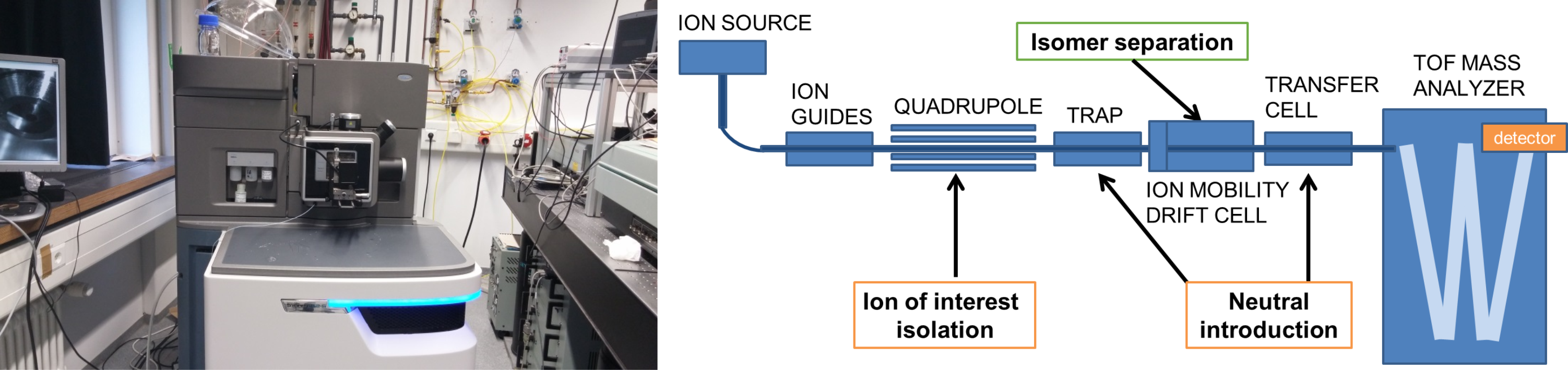
1. Trapped Ion Mobility Spectrometry
The recently (April 2018) installed Bruker timsTOF combines Trapped Ion Mobility Spectrometry (TIMS) with Time-of-Flight mass spectrometry (TOF). Ion mobility is a powerful extension to mass spectrometry that delivers information about the three dimensional structure of an ion and allows for isomer separation.
2. Fourier Transform Ion Cyclotron Resonance
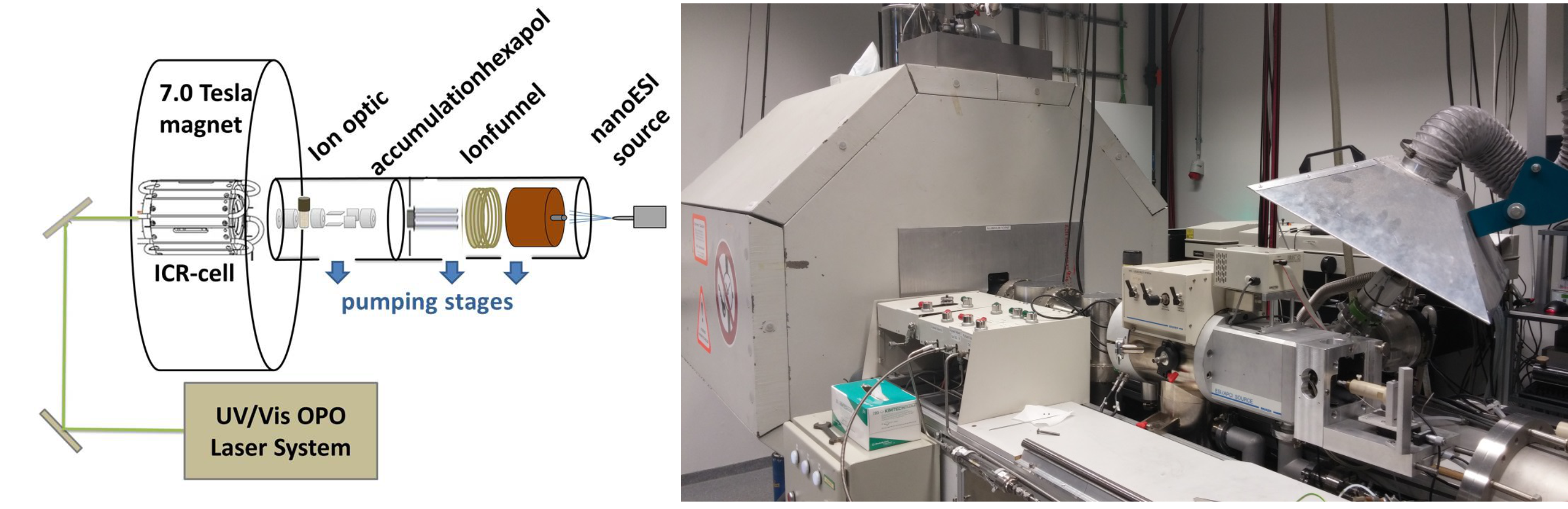
By trapping ions in the magnetic field of a specially modified Fourier Transform Ion Cyclotron Resonance (FT-ICR) mass spectrometer (Bruker Daltonics), we explore the chemistry and photoexcitation of a range of isolated metal-containing complexes, clusters, and other non-covalent species relevant to functional materials. A nano-electrospray ionization (nanoESI) source is used to transfer ionic species of interest from solution to the gas phase, where they can be stored and subsequently: (a) collisionally activated, to study unimolecular fragmentation (b) reacted with neutral species, to study bimolecular behaviors (i.e. selectivity and reaction rate); (b) activated by tunable light (IR and nIR-vis-UV spectral ranges), to generate an optical spectrum.
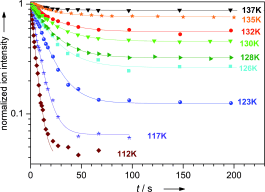
The ICR cell is temperature variable (100 – 400 K) which also enables e.g. the study of association kinetics and binding energies. These experiments provide fundamental chemical and physical insight into the isolated systems, and are valuable benchmarks for computational models.
3. Travelling Wave Ion Mobility
We also utilize Travelling Wave Ion Mobility (TWIMS) to study the properties of isomer-separated ionic species using a Synapt G2 time of flight mass spectrometer (Waters). Ions are generated in an interchangeable ion source (e.g. nanoESI, ESI, MALDI, or ASAP). Prior to mass analysis, the gas phase ions are separated in the TWIMS cell, based on their collisional cross section (CCS), the property describing a molecule’s characteristic topology or “shape and size”. The CCSs derived from experiment are compared to theoretically predicted cross sections for structural identification. In-house modifications to the commercial instrument allow neutral gas introduction in the “trap” and “transfer” regions. As they are separated in time, the isomer-resolved species may have their individual chemistry probed, e.g. (i) via collisional activation to compare fragmentation or (ii) by reaction with neutral species (utilizing the instrumental modification).
We also carry out methodological development on the separation and CCS measurement of metal containing species, large clusters and species possessing non-covalent interactions; systems which currently represent an analytical challenge for the TWIMS technique.
Detection of Intermediates in Dual Gold Catalysis
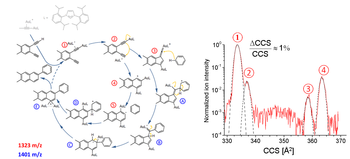
We have probed for reaction intermediates involved in the dual-gold-catalyzed activation of a conjugated 1,5-diyne substrate and its further coupling to benzene in the liquid phase. This was done by sampling the reaction mixture by electrospray ionization followed by high-resolution ion mobility mass spectrometry - under conditions allowing for the resolution of structural isomers differing in their collision cross sections by less than 0.5%. For the cationic mass corresponding to catalyst + diyne (activation stage) we resolve four isomers. At the mass corresponding to catalyst + diyne + benzene, two isomers are observed. By comparing the experimentally obtained cross sections to those inferred for model structures derived from density functional computations, we find our measurements to be consistent with the proposed solution mechanism.
Binding of O2 and CO to Metal Porphyrin Anions in the Gas Phase
The binding energies of O2 and CO to iron(II) and manganese(II) porphyrin anions has been determined in the gas phase. Low-pressure ion–molecule equilibria have been measured in a cryogenically cooled trap of an FT-ICR mass spectrometer, and binding energies of (40.8±1.3) kJ mol−1 and (66.3±2.6) kJ mol−1 have been obtained for oxygen and carbon monoxide, respectively, with a heme-analogue FeII porphyrin complex.
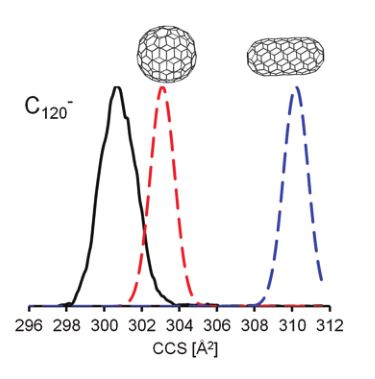
We present high-resolution trapped ion mobility spectrometry (TIMS) measurements for fullerene ions in molecular nitrogen. Three different charge states were studied (monocations, monoanions and dianions) with fullerenes ranging in size from C60 to C150.
PCCP Article
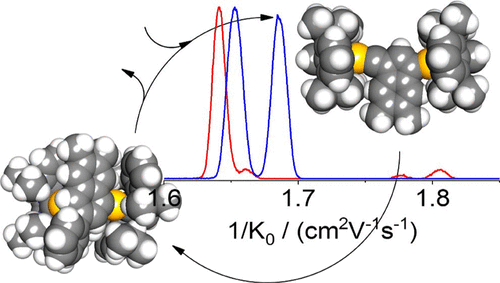
We have probed for reaction intermediates involved in the dual-gold-catalyzed activation of a conjugated 1,5-diyne substrate and its further coupling to benzene in the liquid phase.
Organometallics Article