Materials for Hydrogen Storage
The following content summarizes highlights of the work at INT until 2016.
Meanwhile, the group focuses on new methods and related materials for Electrochemical Storage.
Conversion Materials for Hydrogen Storage
Amide-Imide Systems for Hydrogen Storage
 Amide-imide systems have shown promising results for hydrogen storage. Most importantly, various systems have been shown to be reversible. We have been investigating various combinations of hydrides and contributed to the understanding of the role of dopants and ball milling. Current research focuses on:
Amide-imide systems have shown promising results for hydrogen storage. Most importantly, various systems have been shown to be reversible. We have been investigating various combinations of hydrides and contributed to the understanding of the role of dopants and ball milling. Current research focuses on:
- new materials combinations
- reaction kinetics
- reaction pathways
-
crystal structure
- upscale production
New additive combinations have been tested in order to accelerate hydrogen absorption and desorption.
The data shows that desorption is possible within a few minutes at the working temperature of a high temperature PEM fuel cell (HT-PEM, 170°C), see also U. Ulmer, J.J. Hu, M. Franzreb and M. Fichtner, INT. J. HYD. ENERGY 38 ( (2013) 1439-1449
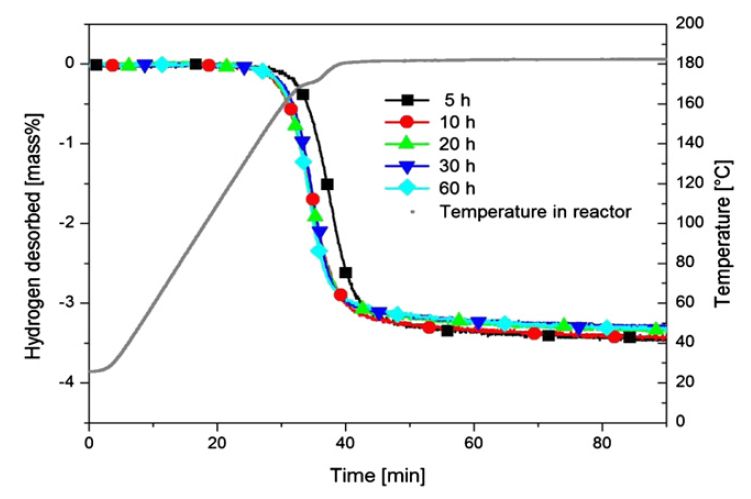
Second desorption of the 2 LiNH2 - 1.1 MgH2 - (0.1 LiBH4 + 3 mass% ZrCoH3) system after ball-milling for 5, 10, 20, 30 and 60 h.
An upscale production of several kg composite material consisting of LiNH2, MgH2 and additives has been succesfully achieved. The material has been integrated and is tested in the worldwide first amide based H storage tank and is currently tested for an APU application in the framework of the EU-RTD project SSH2S:


An IVECO bus has been equipped with an APU which is driven by a HT-PEMFC and an amide tank for hydrogen supply. The amide composite material for the tank was developed and provided by KIT.
Alanate Systems for Hydrogen Storage
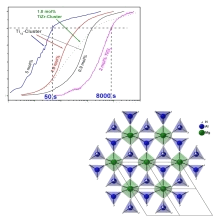
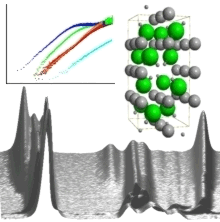
Pure Mg alanate was synthesized in our group and the crystal structure of the compound was determined. We also synthesized Ca alanate and investigated its hydrogen storage behavior. In isothermal kinetic studies we showed for the first time that mass transport is the rate limiting step in the kinetics of the hydrogen ab- and desorption of alanates. In kinetic isotope experiments, AlHx was found to be the mobile species in the materials transformation. We also found that Ti13clusters are world-record catalysts for alanate materials. X ray absorption experiments (EXAFS, XANES) shed light on the development of the near order around the Ti atoms and showed that the dopant forms small Ti-Al clusters during cycling.
Borohydrides for Hydrogen Storage
 Tetrahydroborates or boranates are complex hydrides containing BH4 anions, which have high volumetric and gravimetric hydrogen content. A method for direct synthesis of pure light metal boranate was developed in our group. Pure and adduct free magnesium boranate was synthesized and the hydrogen desorption reaction was studied, see Chlopek, K; Frommen, C; Leon, A; Zabara, O; Fichtner, M, JOURNAL OF MATERIALS CHEMISTRY 17 3496 (2007)
Tetrahydroborates or boranates are complex hydrides containing BH4 anions, which have high volumetric and gravimetric hydrogen content. A method for direct synthesis of pure light metal boranate was developed in our group. Pure and adduct free magnesium boranate was synthesized and the hydrogen desorption reaction was studied, see Chlopek, K; Frommen, C; Leon, A; Zabara, O; Fichtner, M, JOURNAL OF MATERIALS CHEMISTRY 17 3496 (2007)
Moreover, the solid state reaction and catalysis of LiBH4 with Al, Ce and other reactants is a matter of research.
Nanoconfined Systems for Hydrogen Storage
In many hydrogen storage systems it has been shown that using nano sized powders is essential for high reaction kinetics. Infiltrating hydrides into nano structures can be used to control the particle diameter and study size effects of the materials. Infiltration may considerably enhance the kinetics of ab- and desorption. Thermodynamic effects have been found, too. Current research focuses on:
- infiltration and encapsulation methods
- synthesis and investigation of nanocarbon templates
- thermodynamic and kinetic properties
-
microstructural effect
- upscale production
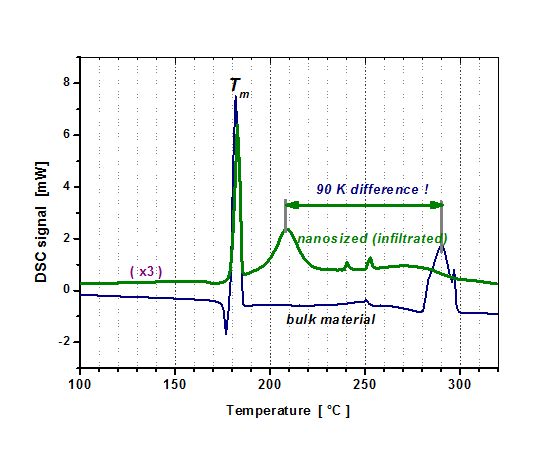
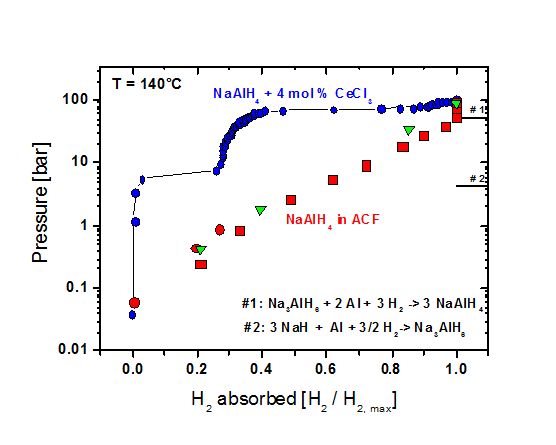
The group has produced and investigated the first composites consisting of a complex hydride infiltrated in microporous carbon template (left). Considerable kinetic and thermodynamic effects (right) were found, see also: R. Prasad, Diploma Thesis, University Darmstadt (2007), W. Lohstroh, A. Roth, H. Hahn, and M. Fichtner, CHEM. PHYS. CHEM. 11, 789 (2010)
![]()
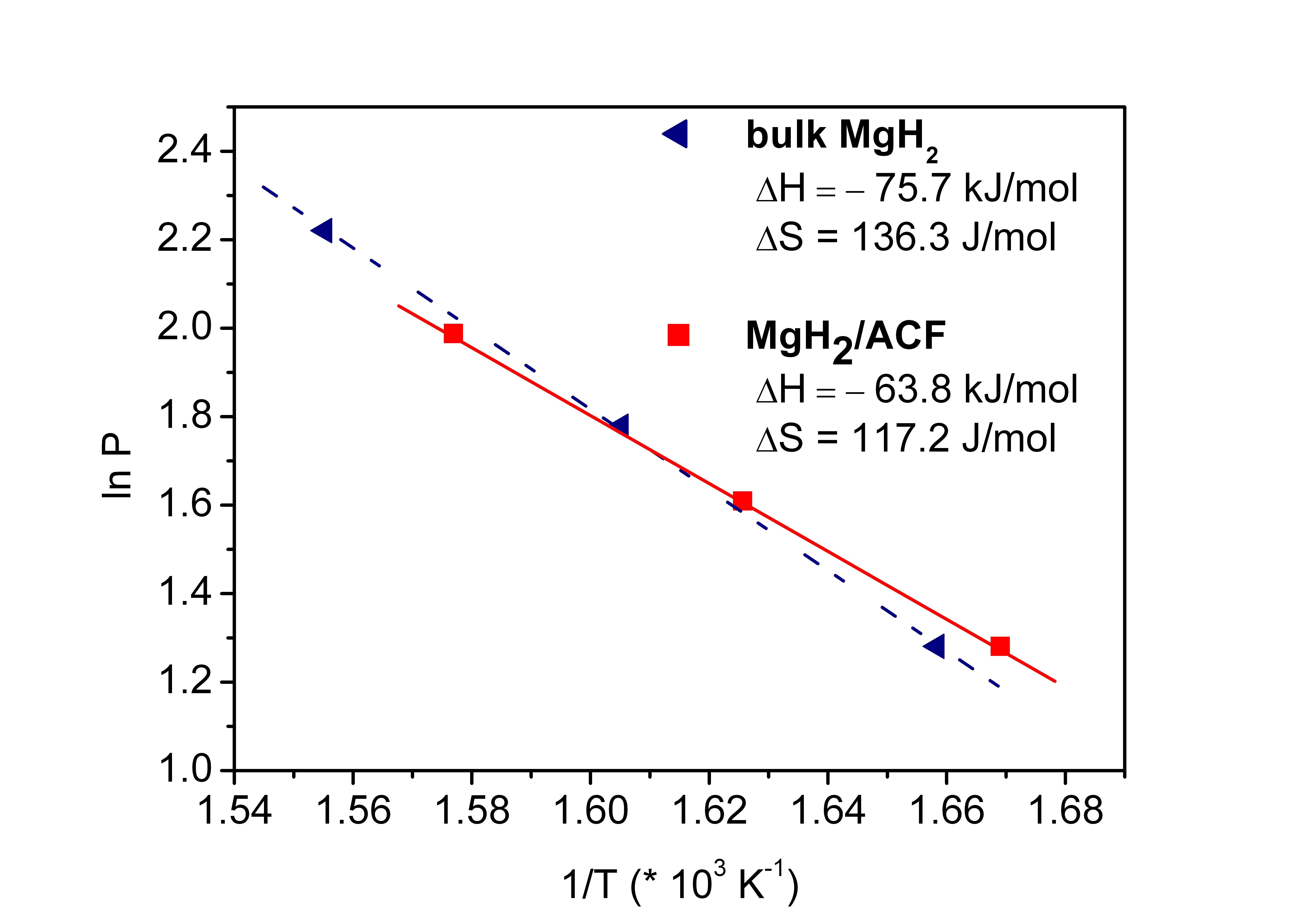
Only when the hydride materials are dispersed at the lower nanoscale, thermodynamic effects can be found. This was also shown with MgH2 infiltrated in microporous carbon. The hydride particles with approx. 1 nm in diameter showed an enthalphy of formation wich was 12 kJ/mol below that of the bulk material. At the same time, the reaction entropy was increased, see also Zh. Zhao-Karger, JJ. Hu, A. Roth, D. Wang, Ch. Kübel, W. Lohstroh and M. Fichtner, CHEM. COMM. 46, 8353-8355 (2010).
Safety Tests
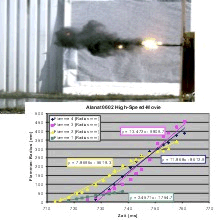 For the first time, scoping studies were performed in order to assess the behaviour of nanoscale Na-alanate powder in contact with various environments. The experiments were small scale device failure tests of alanate-filled tubular tanks, monitored by high speed and IR cameras, fast p and T sensors.
For the first time, scoping studies were performed in order to assess the behaviour of nanoscale Na-alanate powder in contact with various environments. The experiments were small scale device failure tests of alanate-filled tubular tanks, monitored by high speed and IR cameras, fast p and T sensors.
Computational Methods
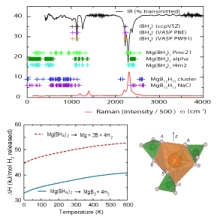
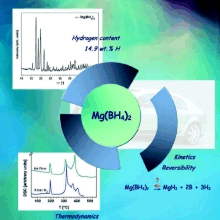
Combining computational techniques like density theory and experimental work has in the past shown to greatly benefit the research. currently in our group DFT is used to help to understand the decomposition of Mg(BH4)2 see also: M.J. van Setten, W. Lohstroh, and M. Fichtner, JOURNAL OF MATERIALS CHEMISTRY 19 7081 (2009)
- Thermodynamics of the decomposition of Mg(BH4)2
- Identification of intermediate species during decomposition of Mg(BH4)2
