Degradation Mechanism Analysis of Lithium-Ion Battery Full-Cells
Our research goals are to identify and understand the degradation processes taking place in full-cells using Ni-rich cathodes during long-term operation in order to tailor the chemistry of cell components and improve the overall performance of LiMO2/graphite-based LIBs.
Advanced Materials Characterization
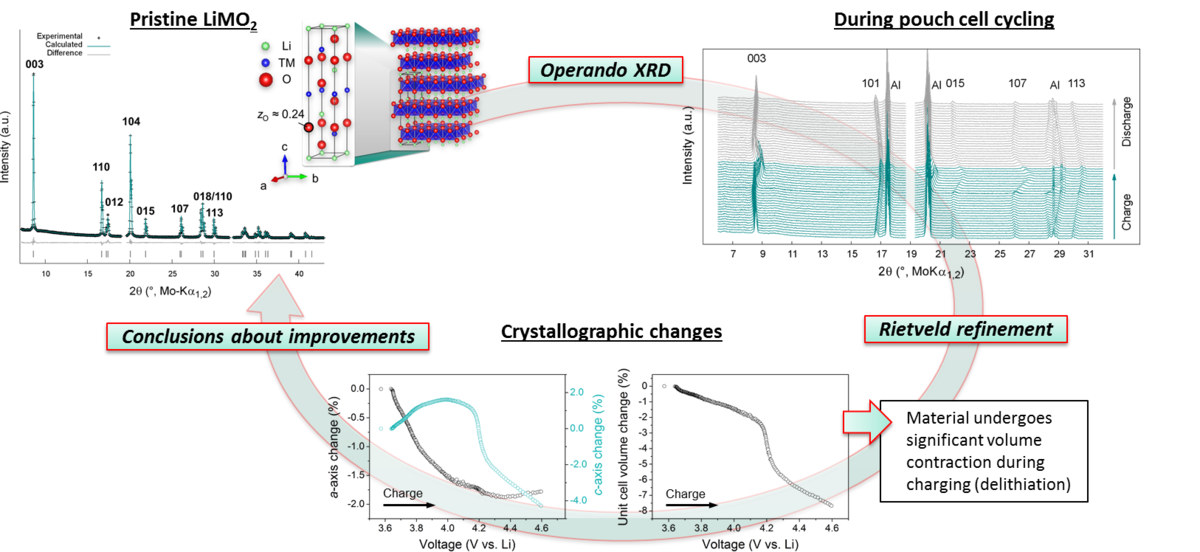
Important deactivation pathways are phase transformations and crystallographic volume changes in the active materials that lead to particle fracture and formation of highly reactive surface. The structural changes of the electrode materials such as evolution of lattice parameters and unit cell volume are analyzed during cell cycling by use of operando X-ray diffraction. In combination with galvanostatic charge/discharge measurements, optimal cycling conditions for specific cathode compositions can be assessed and structure-related long-term aging effects are identified.
Interlinked Ageing Effects Between Cathode and Anode
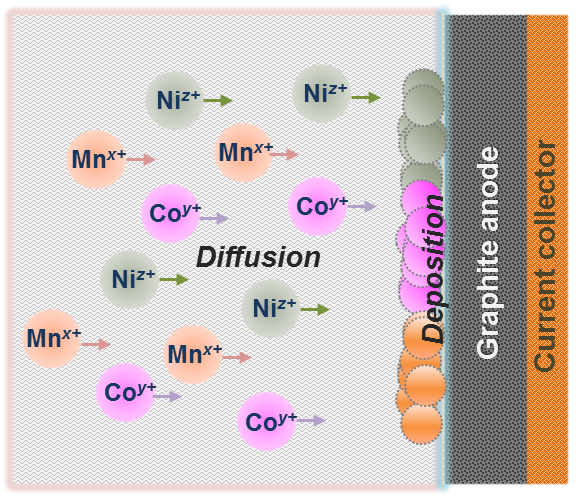
In addition, our studies seek to understand the different electrochemical mechanisms involved in the modification of the anode/electrolyte interface. In particular, the leaching of transition metals (TMs) from the cathode matrix is of interest. TM ions diffuse through the separator and subsequently deposit at the graphite anode, thus disturbing the lithium intercalation. An in-depth evaluation is carried out using three-electrode measurements in order to interpret the processes occurring at cathode and anode separately. Moreover, inductively coupled plasma mass spectrometry, time-of-flight secondary ion mass spectrometry, and transmission electron microscopy are used to characterize the graphite and reveal the location of the TM enrichments.
Bulk Doping and Coating of Cathode Active Materials
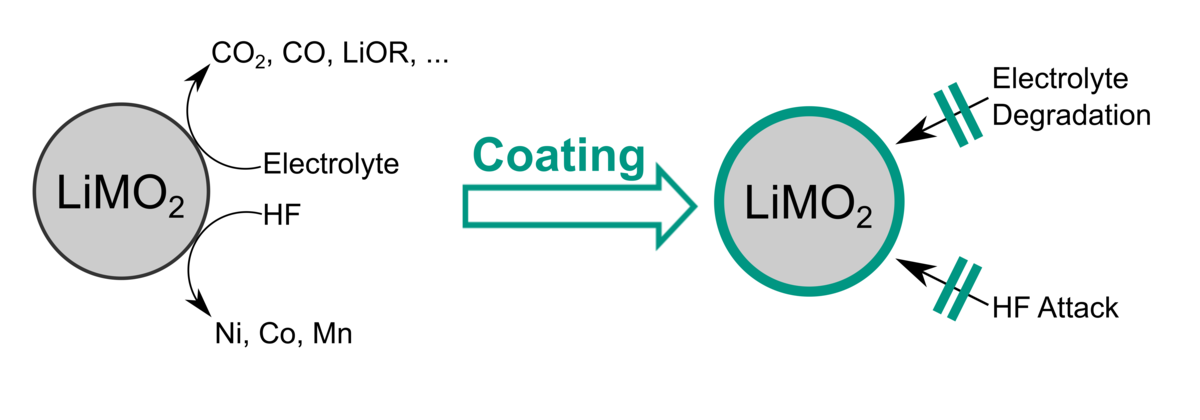
A simple way to improve the performance of layered LiMO2 cathode materials is to dope and/or coat them. To this end, we use both solution chemistry strategies and atomic layer deposition (ALD). The modified materials are characterized using state-of-the-art techniques and their electrochemical performance is evaluated in long-term cycling experiments. Ex-situ analysis of cycled cells allows for better understanding of the prevailing degradation mechanisms and how protective coatings and specific dopants affect/alter these processes.