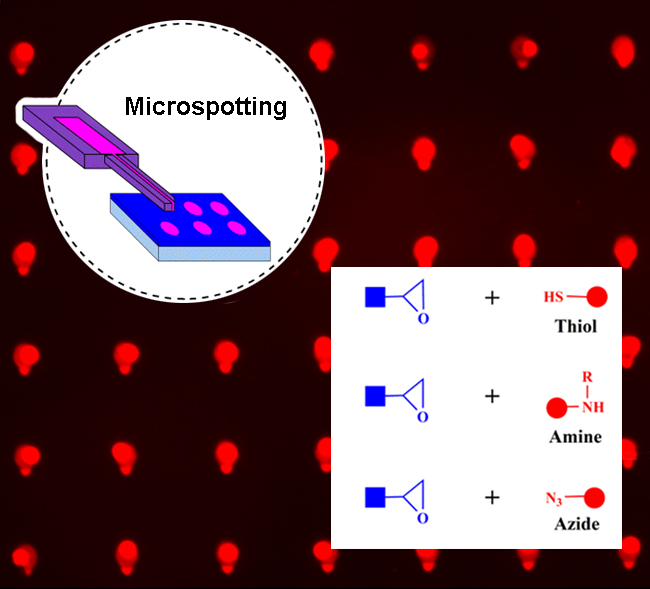
Protein Microarray Immobilization via Epoxide Ring‐Opening by Thiol, Amine, and Azide
-
Author:
S. M. M. Dadfar, S. Sekula‐Neuner, V. Trouillet, M. Hirtz
-
Source:
Adv. Mater. Interfaces 8 (2021) 2002117
- Date: 2021
-
Immobilization of functional molecules in ordered microarrays has vast importance for many applications in sensing and diagnostics. Here, the immobilization of small molecule compounds and protein in microchannel cantilever spotting (μCS) on epoxy‐terminated glass surfaces is studied via ring‐opening of epoxides by thiol, amine, and azide, with the purpose of creating microscale patterns for sensing applications. For this, glass surfaces carrying epoxy groups from silanization are functionalized with microarrays by μCS with different fluorescent and nonfluorescent dyes containing thiol, amine, or azide groups. Experiments with the fluorophores reveal that all routes result in successful immobilization in a short time. Furthermore, from these experiments, optimal reaction conditions including time, temperature, catalyst type, and catalyst amount are determined. The feasibility of these routes in sensing applications is examined by means of protein binding experiments. Comparing the fluorescence intensity values of different biotin carrying spot arrays immobilized on the epoxy‐terminated glass surfaces, after incubating the surfaces with fluorescent‐labeled streptavidin, reveal the highest surface density of immobilized biotin for the amine route in comparison to the thiol and azide routes. The obtained results from this work can inform the design and fabricating of protein biosensors as well as other biomedical and diagnostic applications.
