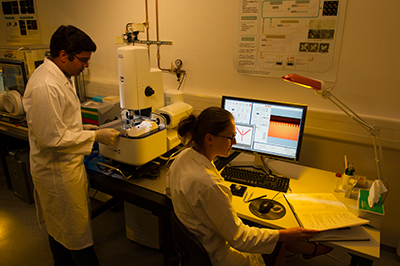
User Facility / KNMFi
We offer access to the state of the art machines for performing surface nano- and microstructuring with techniques like dip-pen nanolithography (DPN), polymer pen lithography (PPL), and related techniques. For sample characterization we have upright and inverted fluorescence microscopes and a SEEC setup for the optical analysis of nanometer-scale thin films. Surface analysis can be obtained by atomic force microscopy (AFM). Furthermore, a FluidFM setup and additional self-built SPL setups are available. Being part of the Karlsruhe Nano Micro Facility (KNMFi), our machines and expertise are available to external users worldwide.
When you are interested in setting up a KNMFi project, don't hesitate to contact us for discussions and submit your idea to the KNMFi proposal system.
Self-inking Stamps
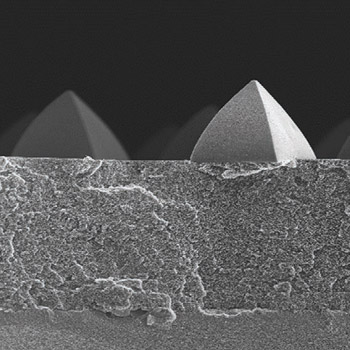
Classical microcontact printing and polymer pen lithography (PPL) involve ink transfer to substrates using solid elastomeric stamps. Ink depletion thus limits the number of successive stamping steps without reinking. Porous stamps developed to overcome this limitation are used only for manual proof‐of‐principle experiments. Here, porous composite stamps for scanner‐based capillary stamping (SCS) that can be mounted on automated printing devices designed for PPL are developed. Porous SCS composite stamps consist of a rigid controlled porous silica glass (CPG) layer and a porous polymeric stamping layer. The latter can be topographically structured with contact elements by replication molding. The mechanical stabilization by the CPG layer ensures that the contact elements are coplanar. SCS allows automated, continuous, high‐throughput patterning enabled by ink supply through the porous SCS composite stamps. Even after more than 800 consecutive stamp–substrate contacts without reinking (the porous SCS composite stamps themselves are used as ink reservoirs), ink microdroplets are deposited without deterioration of the pattern quality. However, SCS also allows supply of additional ink during ongoing stamping operations through the pore systems of the porous SCS composite stamps. SCS can easily be adapted for multi‐ink patterning and may pave the way for further upscaling of contact lithography.
Publication
Scanner‐Based Capillary Stamping
P. Hou, R. Kumar, B. Oberleiter, R. Kohns, D. Enke, U. Beginn, H. Fuchs, M. Hirtz, M. Steinhart
Adv. Funct. Mater. 30 (2020) 2001531, DOI:10.1002/adfm.202001531
Reactive Anti-Fouling Surfaces
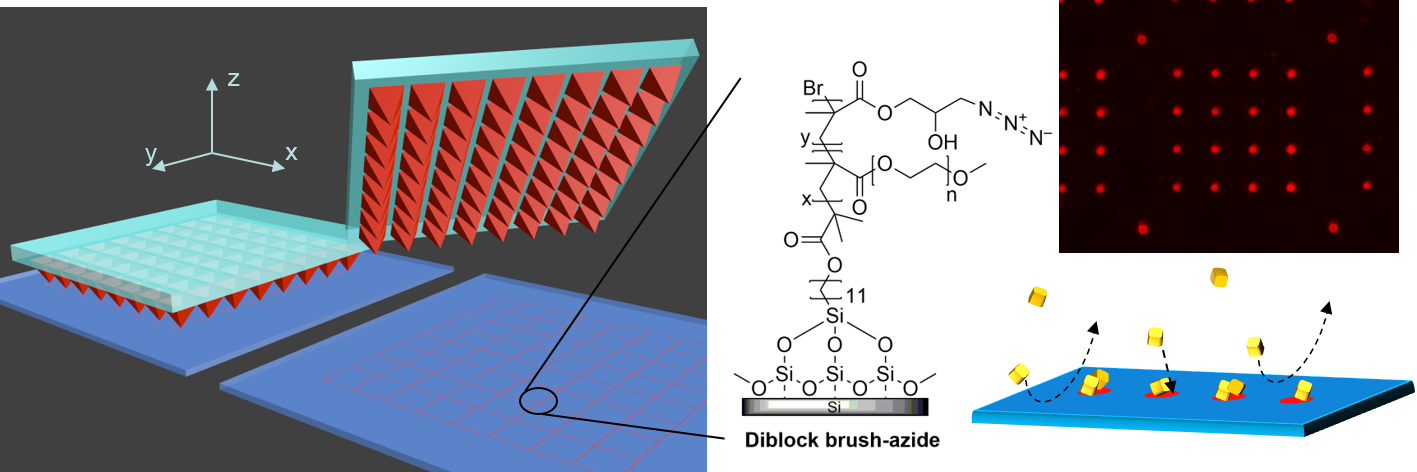
Protein-repellent reactive surfaces that promote localized specific binding are highly desirable for applications in the biomedical field. Nonspecific adhesion will compromise the function of bioactive surfaces, leading to ambiguous results of binding assays and negating the binding specificity of patterned cell-adhesive motives. Localized specific binding is often achieved by attaching a linker to the surface, and the other side of the linker is used to bind specifically to a desired functional agent, as e.g. proteins, antibodies, and fluorophores, depending on the function required by the application. We present a protein-repellent polymer brush enabling highly specific covalent surface immobilization of biorecognition elements by strain-promoted alkyne-azide cycloaddition click chemistry for selective protein adhesion. The protein-repellent polymer brush is functionalized by highly localized molecular binding sites in the low micrometer range using polymer pen lithography (PPL). Because of the massive parallelization of writing pens, the tunable PPL printed patterns can span over square centimeter areas. The selective binding of the protein streptavidin to these surface sites is demonstrated while the remaining polymer brush surface is resisting nonspecific adsorption without any prior blocking by bovine serum albumin (BSA). In contrast to the widely used BSA blocking, the reactive polymer brushes are able to significantly reduce nonspecific protein adsorption, which is the cause of biofouling. This was achieved for solutions of single proteins as well as complex biological fluids. The remarkable fouling resistance of the polymer brushes has the potential to improve the multiplexing capabilities of protein probes and therefore impact biomedical research and applications.
Publication
Clickable Antifouling Polymer Brushes for Polymer Pen Lithography
U. Bog, A. de los Santos Pereira, S. L. Mueller, S. Havenridge, V. Parrillo, M. Bruns, A. E. Holmes, C. Rodriguez-Emmenegger, H. Fuchs, M. Hirtz
ACS Appl. Mater. Interfaces 9 (2017) 12109-12117, DOI:10.1021/acsami.7b01184
Induced Protein Crystallization
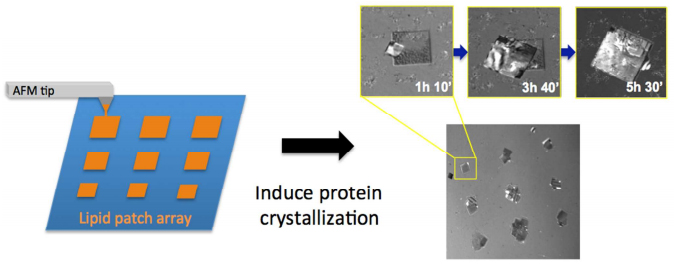
We demonstrate the use of dip-pen nanolithography (DPN) to crystallize proteins on surface-localized functionalized lipid layer arrays. DOPC lipid layers, containing small amounts of biotin-DOPE lipid molecules, were printed on glass substrates and evaluated in vapor diffusion and batch crystallization screening setups, where streptavidin was used as a model protein for crystallization. Independently of the crystallization system used and the geometry of the lipid layers, nucleation of streptavidin crystals occurred specifically on the DPN-printed biotinylated structures. Protein crystallization on lipid array patches is also demonstrated in a microfluidic chip, which opens the way toward high-throughput screening to find suitable nucleation and crystal growth conditions. The results demonstrate the use of DPN in directing and inducing protein crystallization on specific surface locations.
Publication
Dip-Pen Nanolithography Assisted Protein Crystallization
F. S. Ielasi, M. Hirtz, S. Sekula-Neuner, T. Laue, H. Fuchs, R. G. Willaert
J. Am. Chem. Soc. 137 (2015) 154-157, DOI:10.1021/ja512141k