Multinary Chalcogenido Metalates
Alkali metal salts of chalcogenidometalates as innovative Li+ or Na+ superion conductors
Certain alkali metal salts of chalcogenido(semi)metalate anions feature a very high alkali metal ion mobility. Therefore, they are discussed as possible candidates for electrolytes in all-solid-state batteries. The Dehnen Group presented excellent Li+ superion conductors of the general formula LixT1nT2mPyEz (T1 = Al, Si; T2 = Ge, Sn; E = S, Se; Figure 1a, b) that belong to the most efficient superion conductors known to date.[1−3] More recently, this was extended to Na+ superion conductors. Similarly high ion conductivities were measured in these systems (up to 4 mS/cm for Na11Sn2PS12). As confirmed by bond valence site energy (BVSE) calculations, this is due to Na+ vacancies in the crystal structure, which effectively interconnect ion migration pathways in a 3D manner (Figure 1c−1e).[4,5] Such phases are often highly sensitive to hydrolysis, however, which we currently address by systematic iso- or aliovalent substitutions. Further developments are underway to increase the electrochemical stabilities of the electrolytes according to theoretical predictions, and to find resource-efficient ways of the production.[6]
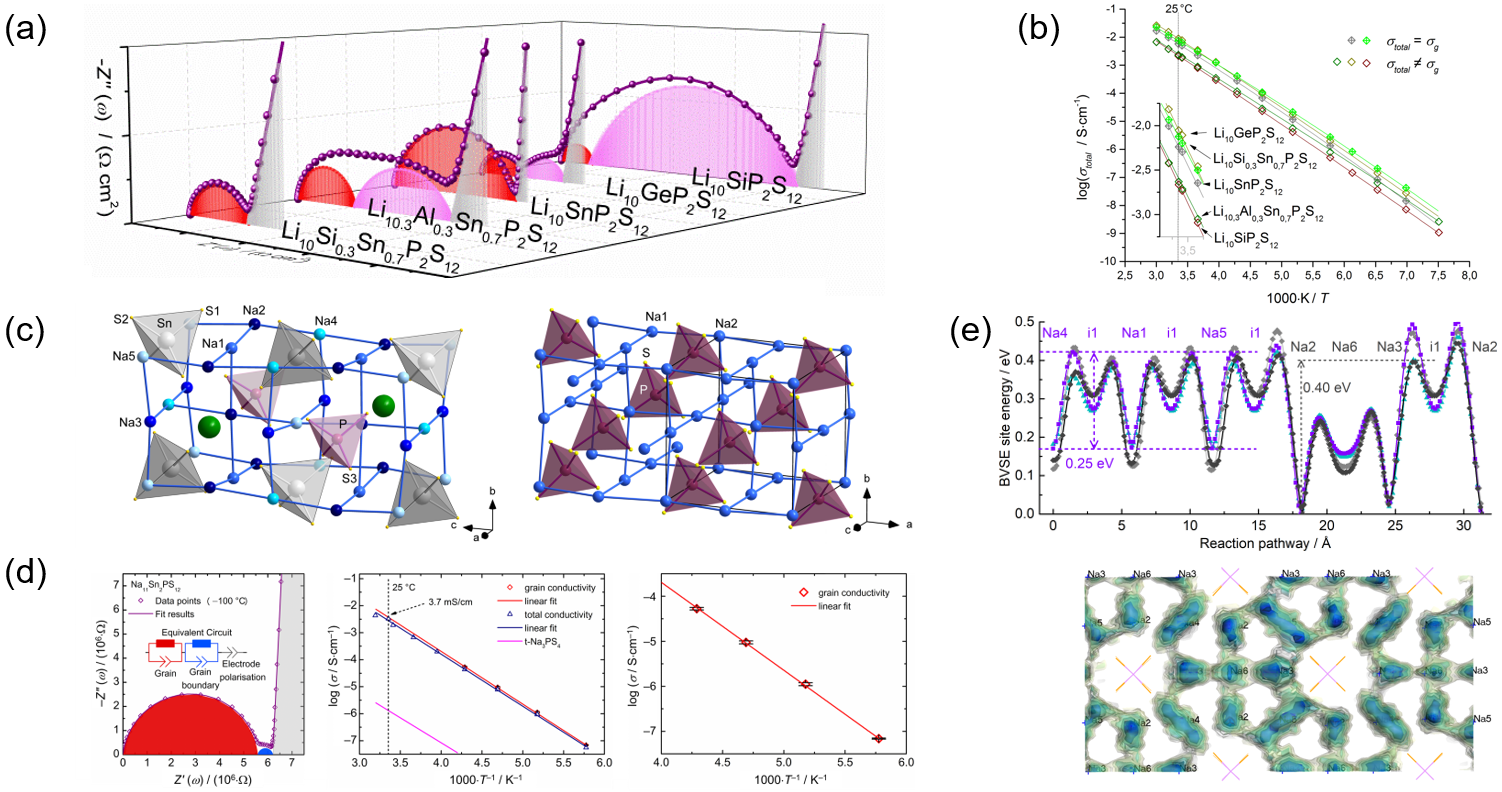
the related Na3PS4 (right). (d) Impedance spectrum and Arrhenius plots of Na11.1Sn2.1P0.9S12. (e) Bond valence site energy (BVSE) models of migration barriers for various relaxed local
structure models of Na11.1Sn2.1P0.9Se12 (top) and slices through the Na density distribution from an empirical NVT molecular dynamics (MD) simulations (bottom).
see e.g.: [1] P. Bron, S. Johansson, K. Zick, J. Schmedt auf der Günne, S. Dehnen, B. Roling, J. Am. Chem. Soc. 2013, 135, 15694–15697. [2] P. Bron, S. Dehnen, B. Roling, J. Power Sources 2016, 329, 530–535. [3] P. Bron, B. Roling, S. Dehnen, J. Power Sources 2017, 352, 127–134. [4] M. Duchardt, U. Ruschewitz, S. Adams, S. Dehnen, B. Roling, Angew. Chem. Int. Ed. 2018, 57, 1351–1355. [5] M. Duchardt, S. Neuberger, U. Ruschewitz, T. Krauskopf, W. Zeier, J. Schmedt auf der Günne, S. Adams, B. Roling, S. Dehnen, Chem. Mater. 2018, 30, 4134–4139. [6] M. Duchardt, M. Diels, B. Roling, S. Dehnen, ACS Appl. Energy Mater. 2020, 3, 6937‒6945.
