Organo-Functionalized Metal Chalcogenide Cages and Networks
Chalcogenidometalate-organic hybrid compounds
Chalcogenidometalate polyanions are often stabilized by cations in the (mostly) crystalline materials. Alternatively, such clusters can be prohibited from reactions to bulk materials of binary or ternary chalcogenides by attachment of (functionalized) organic substituents, [(RfT)xEz] (Rf: functionalized organic substituent; T: Si, Ge, Sn; E: S, Se, Te) – with the added consequence of fundamentally varied materials properties.
As an extension of the Dehnen Group’s fundamental investigations of the synthesis and physical properties of clusters with C=O or COOH functionalization of the organic shell, comprehensive studies have been, and are, performed in the direction of the controlled extension of this shell by attachment of further organic molecules via the substituents’ functional groups.[1−4] These works include bridging molecules, among them diamondoid units (Figure 1a).[1] To gain deeper insight into the formation and reorganization pathways of [(RfT)xEy] systems, structural and spectroscopic investigations were combined with theoretical investigations on DFT level (Figure 1b,c).[2]
During our systematic variation of substituents, we recently identified a particularly interesting class of intrinsically amorphous compounds of the general formula [(ReT)4E6] (Re: electron-rich substituent), which is in the position to convert infrared light emitted by a commercially available, low-cost CW laser diode into a broad (white-light) spectrum (Figure 1d−g).[5,6] The properties of these materials can be controlled by variation of the organic groups, Re, as these affect the (dis)order of the molecules in the solid, which is critical for the observed extreme nonlinear optical response. With the onset of long-range order, the unique supercontinuum-generation is replaced by (albeit very efficient) second-harmonic generation. Further variation of the electronic properties can be realized by varying the components of the inorganic cluster core, T and E.[7−9] As these compounds are intrinsically amorphous, this project strongly relies on the combination of high-level analytics with comprehensive quantum chemical studies (Figure 1h,i). One of the current goals is the generation of inks of differently responding clusters for printable data storage.
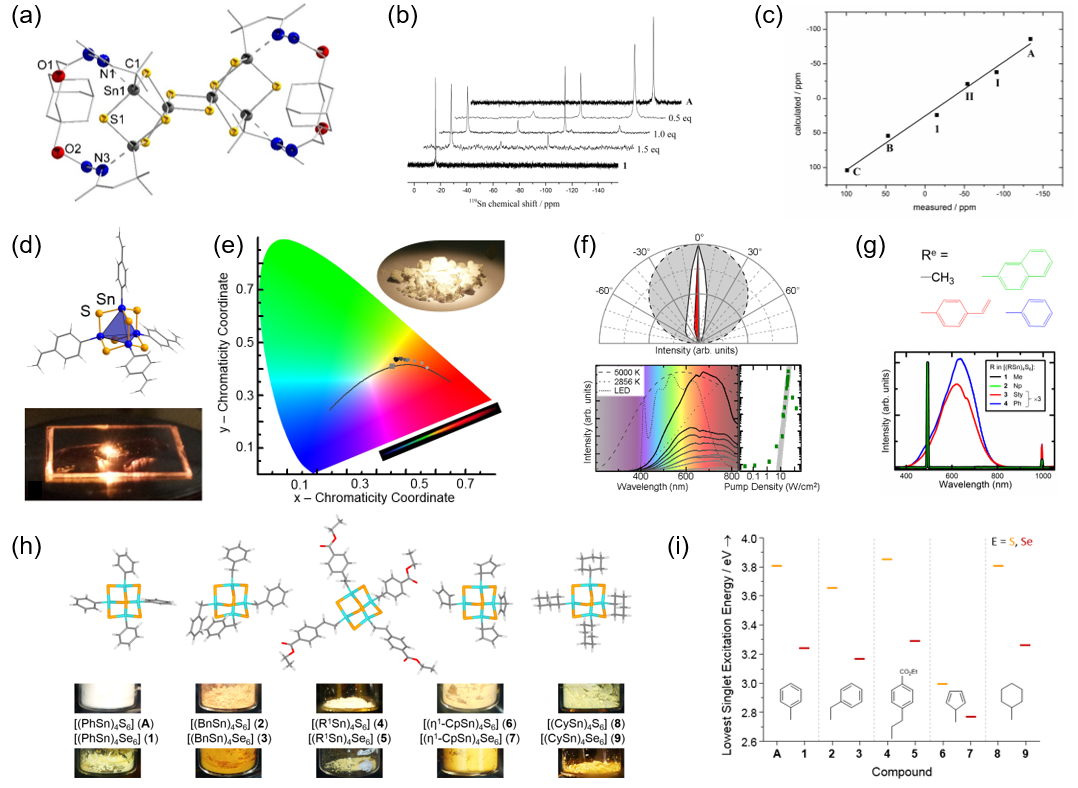
[1] B. Barth, B. A. Tkachenko, J. P. Eußner, P. R. Schreiner, S. Dehnen, Organometallics 2014, 33, 1678–1688. [2] J.P. Eußner, B.E.K. Barth, E. Leusmann, Z. You, N. Rinn, S. Dehnen, Chem. Eur. J. 2013, 19, 13792–13802. [3] J. P. Eußner, R. O. Kusche, S. Dehnen, Chem. Eur. J. 2015, 21, 12376–12388. [4] N. Rinn, J.P. Eußner, W. Kaschuba, X. Xie, S. Dehnen, Chem. Eur. J. 2016, 22, 3094–3104. [5] N.W. Rosemann, J.P. Eußner, A. Beyer, S.W. Koch, K. Volz, S. Dehnen, S. Chatterjee, Science 2016, 352, 1301–1304. [6] N.W. Rosemann, J.P. Eußner, E. Dornsiepen, S. Chatterjee, S. Dehnen, J. Am. Chem. Soc. 2016, 138, 16224–16227. [7] E. Dornsiepen, F. Dobener, N. Mengel, O. Lenchuk, C. Dues, S. Sanna, D. Mollenhauer, S. Chatterjee, and S. Dehnen, Adv. Opt. Mater. 2019, 7, 1801793. [8] E. Dornsiepen, F. Dobener, S. Chatterjee, S. Dehnen, Angew. Chem. Int. Ed. 2019, 58, 17041–17046. [9] K. Hanau, S Schwan, M. R. Schäfer, M. J. Müller, C. Dues, N. Rinn, S. Sanna, S. Chatterjee, D. Mollenhauer, S. Dehnen, Angew. Chem. Int. Ed. 2021, 60, 1176–1186.
Core-shell-shell clusters with transition-metal termination and interaction with metal surfaces
Besides the variation and extension of the organic substituents of binary organtetrel chalcogenide clusters of the type [(RfT)xEy] (Rf: functionalized organic substituent; T: Si, Ge, Sn; E: S, Se, Te; see above), a related research objective pursued by the Dehnen Group is the extension of the inorganic core of such clusters by addition of another metal component, as well as the interaction of a correspondingly modified organic cluster shell with transition metal ions and surfaces.[10] As a result, two types of target compounds are obtained and comprehensively studied:
(A) Onion-type cluster architectures of the general type [(RfT)x(LnM)yEz] (L: ligand; n >= 0; M: transition metal atom), consisting of a transition metal chalcogenide core MyEz, a first shell consisting of TxEz moieties, and a second shell comprising the functionalized organic substituents (Figure 2a),[11−16] which we study in terms of their formation and controlled thermal degradation.
(B) Organotin chalcogenide clusters decorated with substituents Rf that are terminated by chelate ligands (e.g., Rbipy with bipyridine termination) for trapping transition metal ions or transition metal complex fragments from solution (Figure 2b),[17] or clusters [(RpolyarSn)xEz] comprising polyaromatic substituents Rpolyar that allow an interaction with metal or metal-derived surfaces (Figure 2c).[18] Most recently, the extension of a cluster with group 6 metal ions led to the first molecular models of organotin sulfide clusters attached to a transition metal dichalcogenide (TMDC) surface, which was additionally investigated and modelled by quantum chemical methods (Figure 2d).[19]
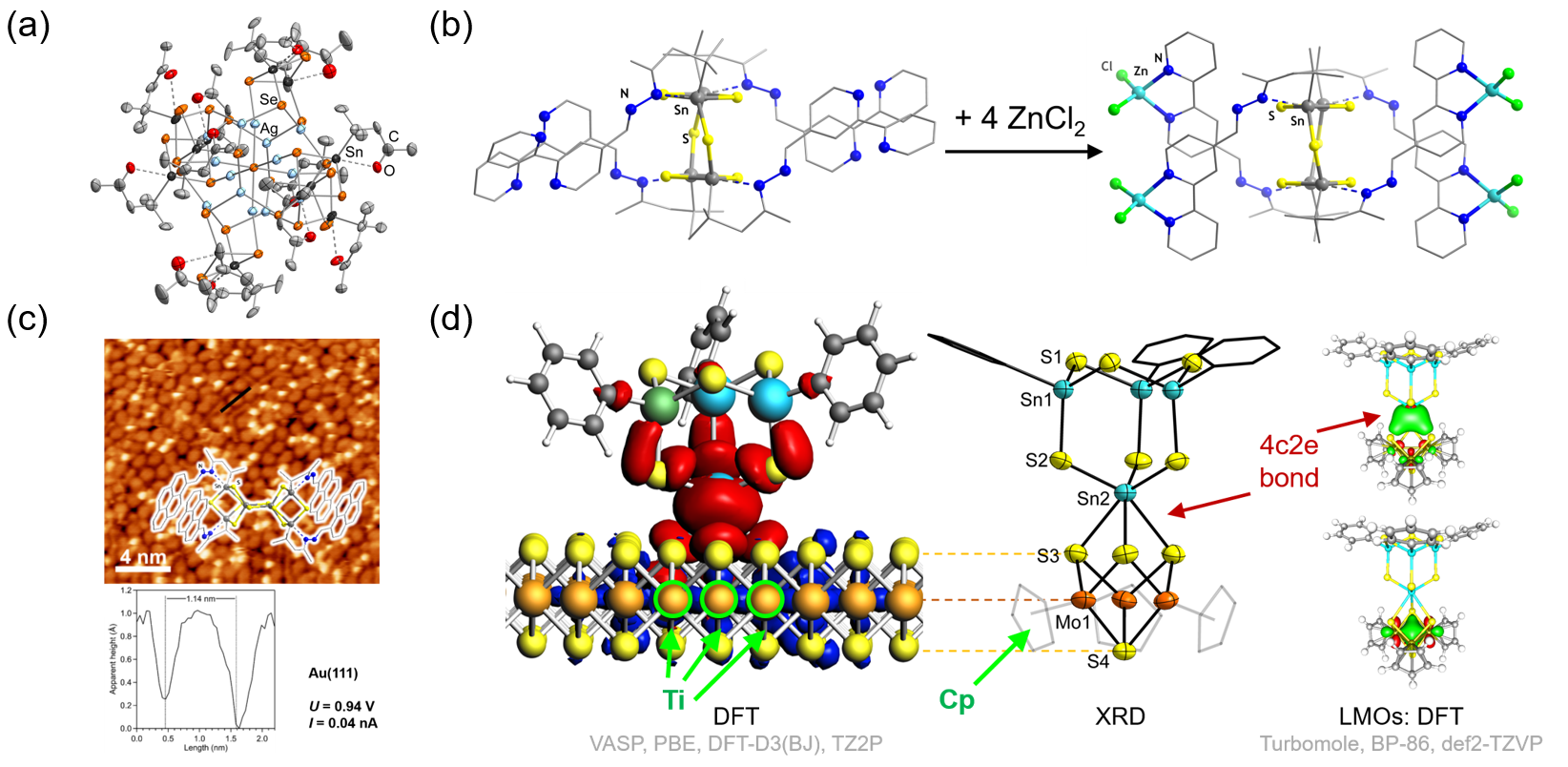
[10] E. Dornsiepen, E. Geringer, N. Rinn, S. Dehnen, Coord. Chem. Rev. 2019, 380, 136–169. [11] E. Leusmann, E. Geringer, B. Weinert, S. Dehnen, Dalton Trans. 2016, 45, 15298–15302. [12] N. Rinn, L. Guggolz, K. Gries, K. Volz, J. Senker, S. Dehnen, Chem. Eur. J. 2017, 23, 15607–15611. [13] N. Rinn, L. Guggolz, J. Lange, S. Chatterjee, T. Block, R. Pöttgen, S. Dehnen, Chem. Eur. J. 2018, 24, 5840–5848. [14] K. Hanau, N. Rinn, M. Argentari, S. Dehnen, Chem. Eur. J. 2018, 24, 11711–11716. [15] N. Rinn, L. Guggolz, J. Lange, S. Chatterjee, T. Block, R. Pöttgen, S. Dehnen, Chem. Eur. J. 2018, 24, 5840–5848. [16] K. Hanau, N. Rinn, S. Dehnen, Inorg. Chem. 2020, 59, 198‒202. [17] E. Geringer, E. Leusmann, F. Tambornino, M. Gerhard, M. Koch, S. Dehnen, Chem. Commun. 2020, 56, 4769‒4772. [18] E. Leusmann, F. Schneck, S. Dehnen, Organometallics 2015, 34, 3264‒3271. [19] E. Dornsiepen, F. Pieck, R. Tonner, S. Dehnen, J. Am. Chem. Soc. 2019, 141, 16494–16500.
Bio-organic functionalization of organotetrel chalcogenide clusters
The attachment of biomolecules to chalcogenide clusters of tetrel elements was initiated and pursued within the LOEWE SynChemBio project, with the aim to provide innovative inhibitors of protein kinase A (PKA), in close collaboration with colleagues from organic chemistry and pharmacy departments. In this context, the decoration of such clusters with amino acids was realized for the first time (Figure 3a,b).[20] Subsequent extensions towards oligopeptides made use of condensation reactions, and also click-chemistry, which allowed for an orthogonal functionalization strategy (Figure 3c,d).[21−24] Current work aims at the use of biomolecules in a way that allows for the clusters’ compatibility with biological environments. For this, pH-dependent hydrolysis and protolysis is studied,[25] with the long-term goal of using the clusters as mobile reservoirs for a controlled release/delivery of cytotoxic H2S in/to undesired cells. As the bulky and highly flexible biochemical groups often hamper crystallization, parallel ESI-MS and DFT studies are undertaken to model the bioorganic wrapping of the cluster cores.
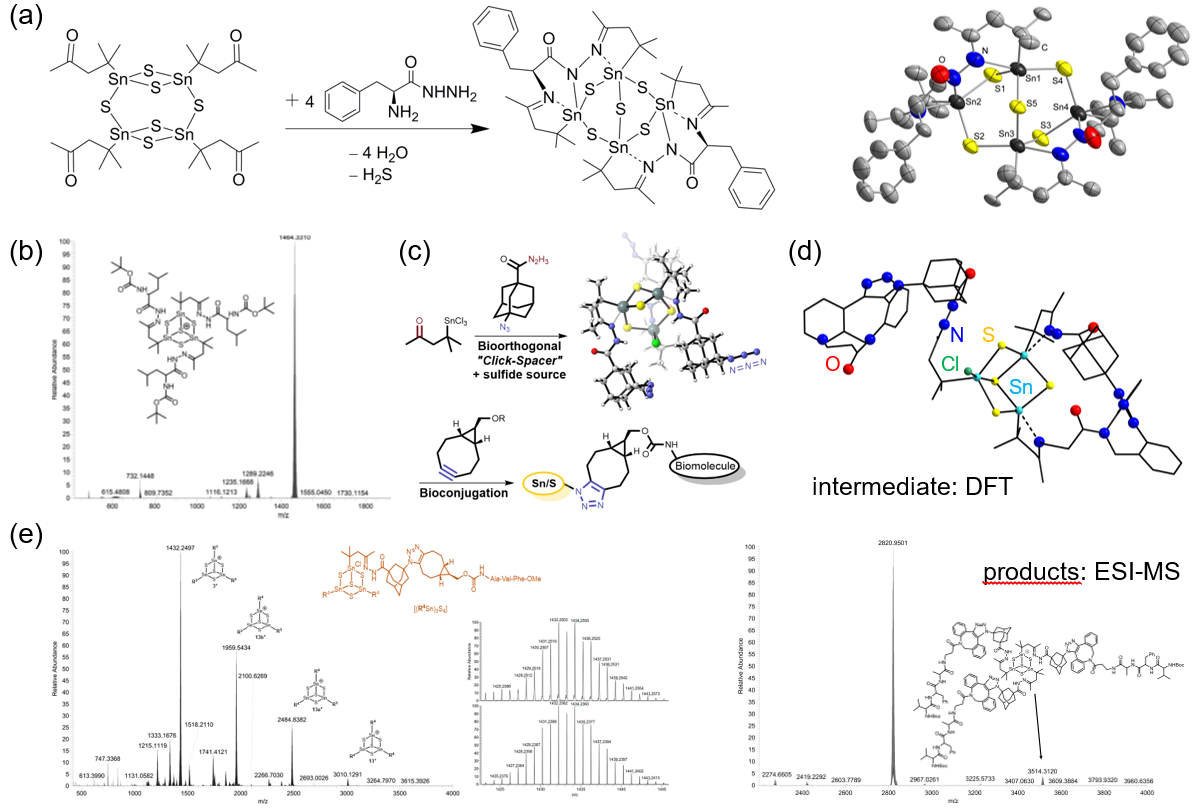
[20] B. E. K. Barth, B. A. Tkachenko, J. P. Eußner, P. R. Schreiner, S. Dehnen Organometallics 2014, 33, 1678–1688. [21] N. Rinn, J.-P. Berndt, A. Kreher, R. Hrdina, M. Reinmuth, P. R. Schreiner, S. Dehnen, Organometallics 2016, 35, 3215–3220. [22] J.-P. Berndt, A. Engel, R. Hrdina, S. Dehnen, P. R. Schreiner, Organometallics 2019, 38, 329–335. [23] A. Engel, E. Dornsiepen, S. Dehnen, Inorg. Chem. Front. 2019, 6, 1973–1976. [24] A. Engel, S. Dehnen, Eur. J. Inorg. Chem. 2019, 4313–4320. [25] A. Engel, H. Dewald, A. Reuter, J. Klippstein, S. Dehnen, Eur. J. Inorg. Chem. 2020, 2809‒2815.
