Non-Classical Chalcogenido Metalates
Ionothermal synthesis of non-classical chalcogenidometalates
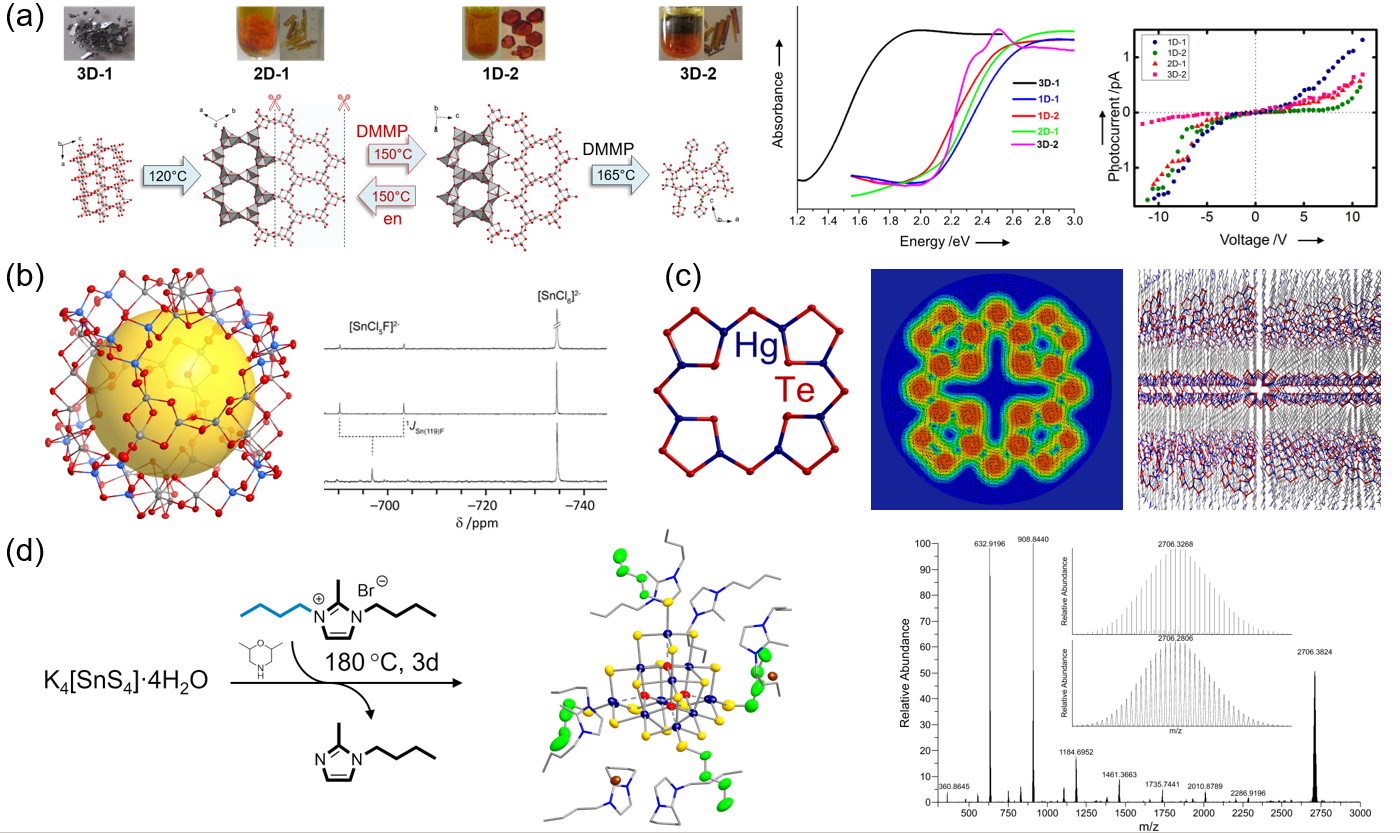
Crystalline chalcogenidometalates of the general type Ax[TyEz] (A = alkali metal cation; T = Ge, Sn; E = S, Se, Te) are synthesized under ionothermal conditions in the Dehnen Group, that is, in ionic liquids under (only) slightly elevated temperatures. Depending on the nature of the cation of the used ionic liquid, and the exact reaction parameters applied, very uncommon chalcogenido metalate architectures can be realized.[1] One important goal is to understand the impact of the reaction conditions on the dimensionality of the anionic substructures and the corresponding opto-electronic conditions of the compounds (Figure 1a).[2–4]
The reaction space is studied in detail, which started out with a primary focus upon the formation of the unique superspherical selenido(semi)metalate anion [Ge24Sn32Se132]24– (Figure 1b).[5] It was possible to determine the key role of Cl− anions during the ionothermal reaction by means of NMR spectroscopy.[6] This anion is the largest known main group element polyanion. Another species worth mentioning is the anion [Hg8Te16]8– with porphine topology (Figure 1c), salts of which crystallize from ionic liquids possessing long alkyl chains at the imidazolium-based cations. Within these salts, anions and cations are arranged in a lamellar manner, which is why these compounds are currently further investigated with the aim of exfoliation.[7]
Very recently, the methylation of chalcogenidometalate clusters was reported for the first time, which has been inhibited so far by the extremely weak nucleophilicity of these species. It was achieved in situ by treatment of corresponding precursors in imidazolium-based ionic liquids.[8–9] It is worth noting that the methylation takes place under mild conditions and by means of the reaction medium itself, hence without the addition of any further (highly toxic) methylation agents. The methylation selectively addresses terminal chalcogenide ligands, which is currently extended and generalized to other, selective alkylations (butylation, decylation). We could recently show that this enables the clusters to dissolve in common solvents (Figure 1d).[10]
also see e.g.: [1] S. Santner, J. Heine, S. Dehnen, Angew. Chem. Int. Ed. 2016, 54, 876–893. [2] Y. Lin, D. Xie, W. Massa, L. Mayrhofer, S. Lippert, B. Ewers, A. Chernikov, M. Koch, S. Dehnen, Chem. Eur. J. 2013, 19, 8806-8813. [3] C. Donsbach, G. Thiele, L. H. Finger, J. Sundermeyer, S. Dehnen, Inorg. Chem. 2016, 55, 6725–6730. [4] S. Santner, J. Sprenger, M. Finze, S. Dehnen, Chem. Eur. J. 2018, 24, 3474–3480. [5] Y. Lin, W. Massa, S. Dehnen, J. Am. Chem. Soc. 2012, 134, 4497-4500. [6] S. Santner, S. Yogendra, J. J. Weigand, S. Dehnen, Chem. Eur. J. 2017, 23, 1999–2004. [7] K. Reiter, D. Sundholm, F. Weigend, S. Dehnen, Angew. Chem. Int. Ed. 2018, 57, 8770–8774. [8] B. Peters, S. Santner, C. Donsbach, P. Vöpel, B. Smarsly, S. Dehnen, Chem. Sci. 2019, 10, 5211–5217. [9] B. Peters, N. Lichtenberger, E. Dornsiepen, S. Dehnen, Chem. Sci. 2020, 11, 16‒26. [10] B. Peters, G. Stuhrmann, F. Mack, F. Weigend, S. Dehnen, Angew. Chem. Int. Ed. 2021, 60, 17622‒17628.
Novel chalcogenidometalates of heavy d10 metals
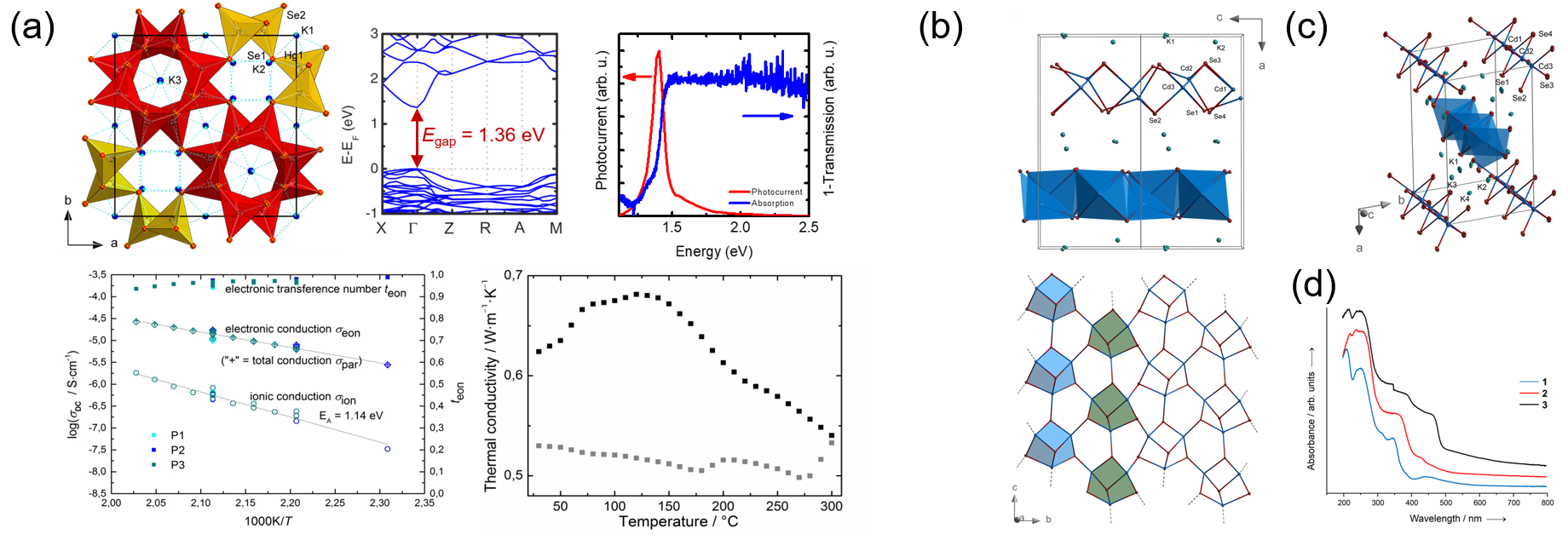
Developments in contemporary materials include heavy elements wherever high atomic masses, high nucleic charges, or flexible interatomic bonds are beneficial or even mandatory for the desired properties. Examples are thermoelectric solids and optical storage materials, which in many cases puts the toxicity of such elements in the rear. Materials research done in the Dehnen Group addresses chalcogenides of cadmium, mercury, lead, and bismuth – with the latter being the most promising owing to its low or non-existent toxicity. The investigations demonstrate the impressive structural diversity of corresponding compounds, which comes along with narrow and finely-tunable band gaps and further interesting physical properties (Figure 2).[11‒15] Current work is dedicated to large-scale synthesis and computer-aided optimization of the compounds’ properties.
[11] G. Thiele, S. Lippert, F. Fahrnbauer, P. Bron, O. Oeckler, A. Rahimi-Iman, M. Koch, B. Roling, S. Dehnen, Chem. Mater. 2015, 27, 4114–4118. [12] G. Thiele, Z. You, S. Dehnen, Inorg. Chem. 2015, 54, 2491–2493. [13] G. Thiele, C. Donsbach, R. Riedel, M. Marsch, K. Harms, S. Dehnen, Dalton Trans. 2016, 45, 5958–5967. [14] G. Thiele, C. Donsbach, I. Nußbruch, S. Dehnen, J. Vis. Exp. 2016, 118, e54789. [15] G. Thiele, P. Bron, S. Lippert, F. Nietschke, O. Oeckler, M. Koch, B. Roling, S. Dehnen, Inorg. Chem. 2019, 58, 4052–4054.
