Electrochemical Properties of High Entropy Materials
High‐entropy materials, especially high‐entropy oxides (HEO), have gained significant interest over the last few years due to their unique structural characteristics and possibility for tailoring of functional properties. These compounds comprise the incorporation of multiple metal cations into single-phase crystal structures and interactions among the various metal cations leading to interesting novel and unexpected properties. Owing to their robust Li-ion storage properties induced by the entropy stabilization effect, transition-metal-based HEO are considered promising materials for use in electrochemical applications, e.g. Li-ion batteries.
The work of our group extends from the synthesis of such novel materials all the way to their integration in full liquid electrolyte based battery cells.
Development of anode materials
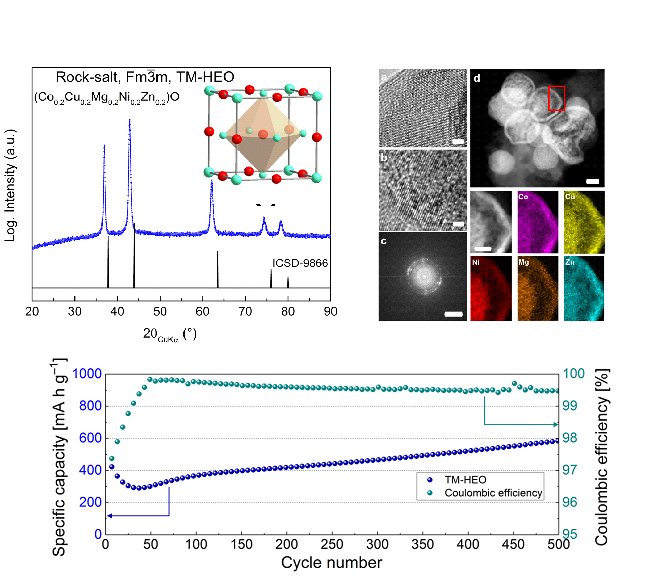
An example is the high-entropy anode material (Co0.2Cu0.2Mg0.2Ni0.2Zn0.2)O (specific capacity: 600 mAhg-1), which was synthesized, processed and successfully cycled. The cycling performance was studied. Additionally, atomic probe tomography (APT) results showed that the constituent cations in all HEO systems were homogenously distributed even at the atomic scale.
Development of cathode materials
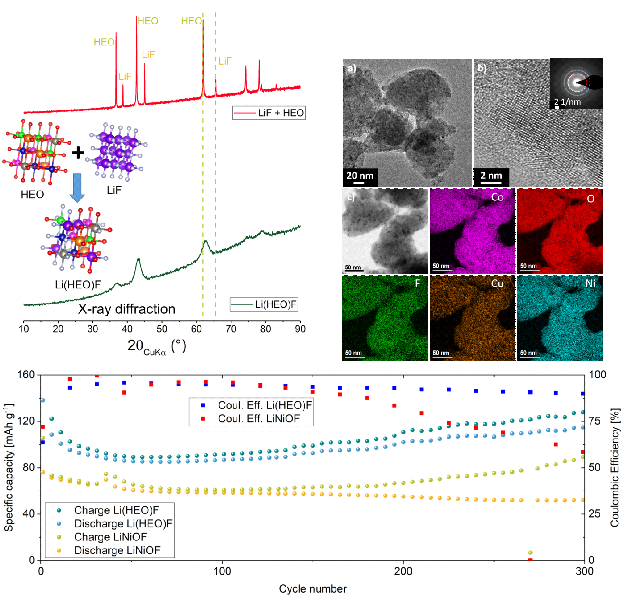
For the application as cathode materials multi-anionic and -cationic compounds are prepared by facile mechano-chemistry using the transition-metal-based HEO as the precursor and LiF as the reactant, leading to formation of lithiated materials. The Li-containing entropy-stabilized oxyfluoride (Lix(Co0.2Cu0.2Mg0.2Ni0.2Zn0.2)OFx) exhibits a working potential of 3.4 V vs. Li+/Li and shows enhanced Li storage properties due to entropy stabilization, compared to conventional oxyfluorides. Additionally, it is observed that the electrochemical behavior of the high entropy oxides depends on each of the metal cations present, thus providing the opportunity to tailor the electrochemical properties by simply changing the elemental composition.
Development of solid electrolytes
The application of the high entropy concept to different solid electrolytes is an emerging research field that would allow the assembly of a solid-state battery cell. Solid electrolytes need to meet several requirements, e.g. a large electrochemical window, chemical stability towards the electrode materials as well as thermal stability. One of the most promising solid electrolytes is the garnet-type oxide electrolyte LLZO. The material was successfully integrated in a battery cell. Established solid electrolyte systems provide grounds for further development by introducing the high-entropy concept.
The diversity of materials design, provided by the entropy‐mediated phase‐stabilization concept, allows engineering of new functional materials for a variety of electrochemical applications, warranting further studies in this emerging field of materials science.