General Features
1. What are High-Entropy Materials?
High-Entropy Materials include many different elements in the same structure and sublattice, which increases the configurational entropy. A high configuratonal entropy can result in different features and can lead to a structural stabilization. Additionally, the interplay between the manifold incorporated elements gives rise to unprecedented materials properties and allows a unique property tailoring.
Pioneering work in 2004 presented a new approach of materials design with the aim "to investigate the unexplored central regions" of multicomponent metallic phase diagrams. It was observed that most of the near-equiatomic multicomponent alloys resulted in a significantly lower number of phases and even single phased alloys compared to the maximum allowed by Gibbs phase rule. The formation of a single phase solid solution can be related to the increased configurational entropy of mixing, stemming from the large number of constituent elements and their respective molar proportions. Thus, the discovery of high-entropy alloys (HEAs) can be credited to two ideas: (a) to investigate unexplored central regions of multicomponent phase diagrams, where unexpected synergies can be anticipated; (b) to precisely control the configurational entropy (by altering the number of elements and/or their proportions) to tailor the phase composition and therefore the properties.
These two design concepts are now extended to other kind of materials, i.e., going beyond metallic HEAs. Since 2004, attempts have been made to investigate non-metallic high-entropy materials (HEMs) like nitrides and carbides. However, a major leap in the research interest on non-metallic HEMs has been observed, following the first report on high-entropy oxides (HEOs) by Rost et al. in 2015. Since then, HEOs have gained significant research attention resulting in numerous compositions, crystal structures, microstructures, and most importantly, tailorable properties. Many HEO compositions are known to exhibit exceptional properties. Research activities on HEOs are essential, due to the still limited knowledge, which is by far not sufficient to predict their overall potential. Additionally, numerous fundamental questions are yet to be clarified, for instance, the driving force behind the phase stability, the specific role of entropy, the underlying mechanisms governing the properties, the effect of the individual cations, etc.
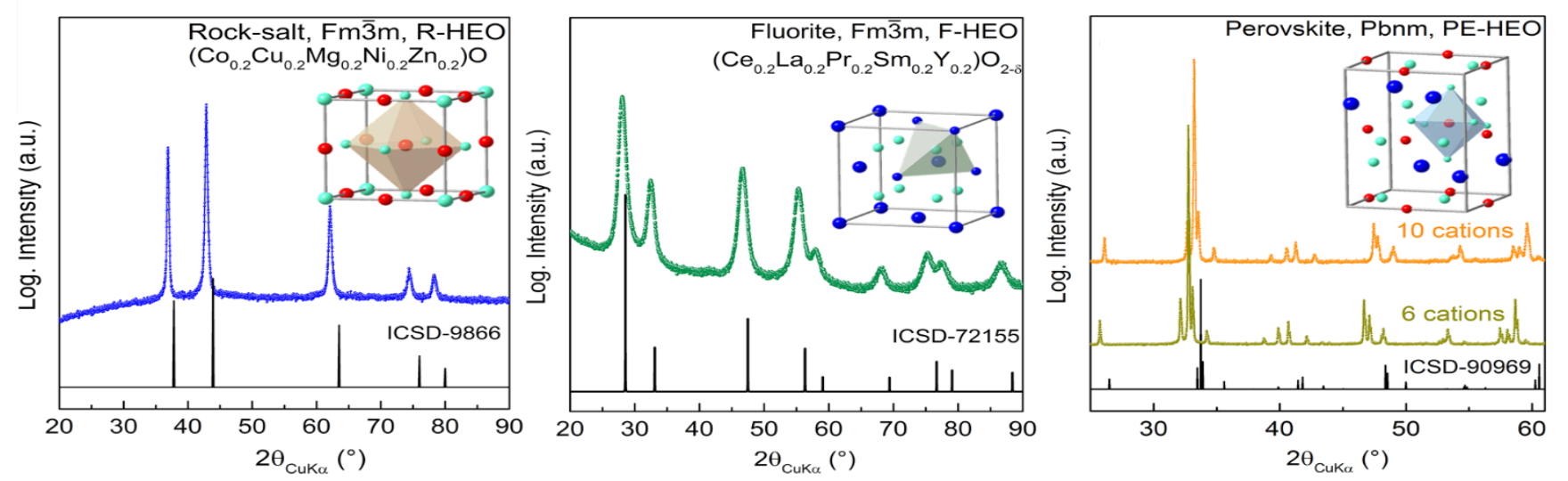
The High-Entropy Materials group has outstanding expertise with the synthesis of a wide range of compositions and structures, e.g., several transition metal HEOs showing a rock-salt structure and, for the first time, fluorite-type rare earth HEOs. When combining transition and rare earth HEOs a single phase perovskite structure can be achieved.
2. The Key to HEM: Entropic Stabilization
The high entropy concept includes the incorporation of many different elements into a single phase structure. Our group focuses on the effects and features arising from this concept and tries to develop novel materials for a variety of different applications. Countless possible combinations and stoichiometries, arising from the combinatorial nature of the high entropy concept, allow to design high entropy materials and to tailor their properties by simply exchanging elements or by changing the stoichiometry. This great versatility in composition together with the features arising from the structure/property relationship of materials, render the high entropy materials as a very exciting materials class with many different areas of applications.
Besides "just" showing a high entropy, some compositions can also show entropy stabilization of the crystal structure. This entropy stabilization can appear when the entropy dependent term (2) in the Gibbs-Helmholtz equation compensates the mixing enthalpy (1), which arises when different elements are mixed.
(1) ΔG = ΔH - TΔS
The part of entropy, which is important in the high entropy concept, is the configurational entropy (Sconfig). Sconfig solely depends on the number of incorporated different elements and can be calculated using a Boltzmann derived equation:
(2) $${ S_{config}~=~-R \left[ ~x~\left( \sum_{a=1}^{M}{x_a \ln{x_a}} \right)_{A-site}~+~y~\left( \sum_{b=1}^{N}{y_b \ln{y_b}} \right)_{B-site}~+~z~\left( \sum_{o=1}^{P}{z_o \ln{z_o}} \right)_{O-site}~\right] }$$
Synthesis Methods
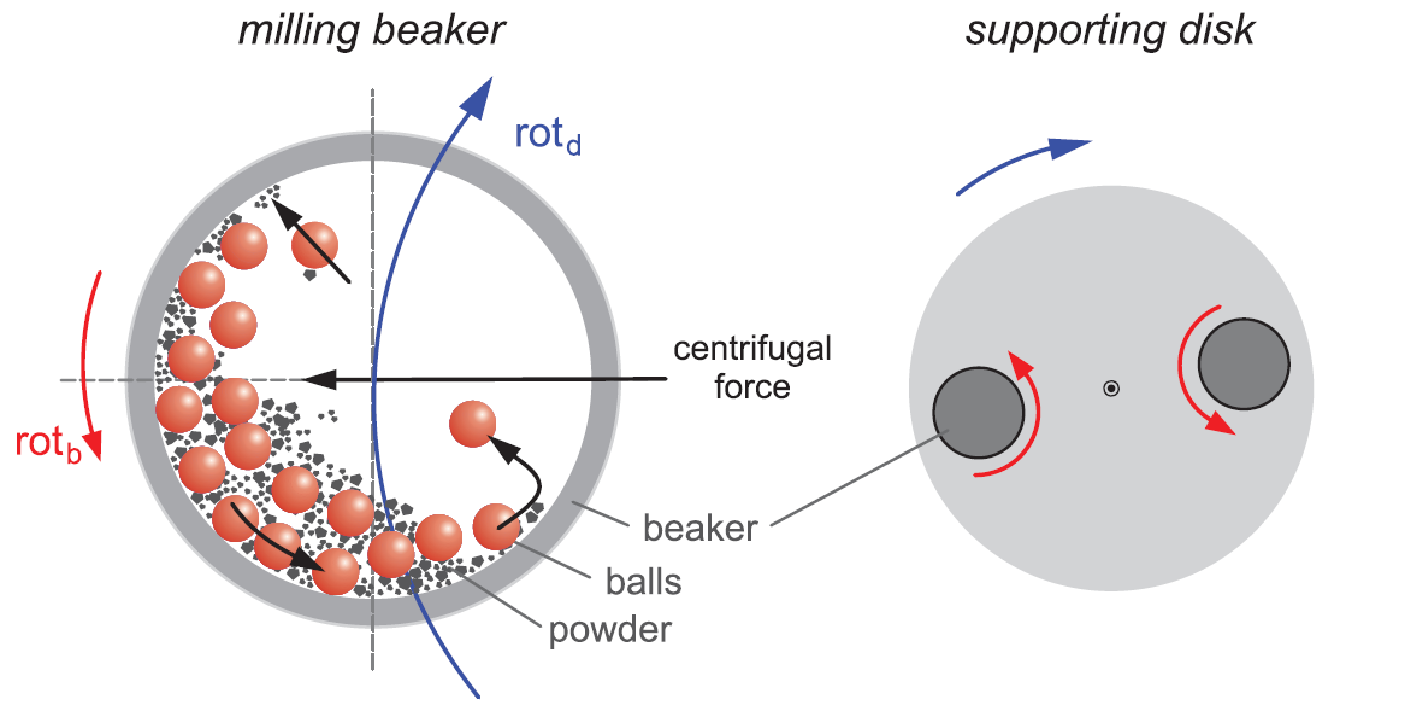
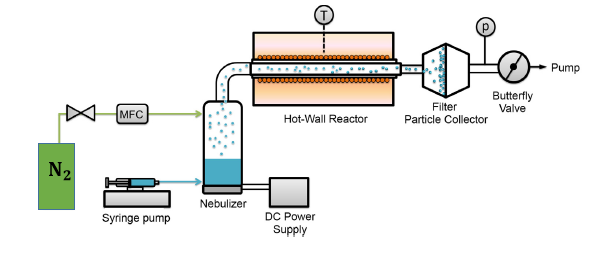
Different high entropy material systems have been synthesized in our group using a variety of techniques, such as mechanosynthesis, reverse co-precipitation, flame spray synthesis and nebulized spray pyrolysis (NSP). NSP has been shown to be the most versatile technique, in terms of flexibility in changing the composition and in obtaining well-crystalline nanostructured materials in the as-prepared state, i.e. without any post-synthesis annealing.
There are in fact numerous research possibilities using HEMs, as the synergies arising from the presence of multiple elements can practically affect all the properties associated with metal oxides. However, it is rather obvious that out of all the studied properties only a few will be highly influenced by this extreme compositional complexity and the resulting synergies. Nevertheless, the topic is still in its infancy, hence, a broad range of properties need to be investigated to comprehend the full potential of HEMs. Given their compositional immensity, a multitude of structural and chemical combinations are possible. Hence, experimentally scavenging the “sweet-spot” for specific purposes will be rather tedious and probably in many cases disappointing. Theoretical approaches like CALPHAD, MD simulations and density functional theory (DFT) calculations can provide a basic guideline for the phase equilibria, structure and (some) properties of the HEMs. However, traditional simulation approaches might still be tedious for systems with such complexity. Hence, combinatorial synthesis and high-throughput approaches with proper descriptors, can accelerate the identification or discovery of new HEOs with specific properties. Certainly, a more active cooperation between the experimental and theoretical researchers will be crucial for the advancement of HEMs. Properties of materials can be classified into “synergetic” properties, when the interplay between the incorporated elements is the most important factor, or in “single element driven” properties, when a specific element in the multicomponent system is responsible for the properties. Regardless, of the underlying mechanisms, all these materials deserve further investigations, since the promises they offer are countless.