High-Entropy Materials (HEM)
The High-Entropy Materials group works on the comprehensive understanding of the high-entropy concept and on the utilization of high entropy materials (HEM) for different applications. Due to the versatility of HEM regarding composition and the connected structure/property relationships, a multitude of different areas of applications is conceivable.
Besides general investigations about the high-entropy concept itself and how to predict propterties in HEM, more application oriented topics are in our focus of interest and presented more in detail on the respective topic pages below. They include applications in electronic devices and energy storage, catalysis, band structure tailoring, and mangetism, as well as method development of high-throughput synthesis and analysis.
Our Research on High Entropy Materials
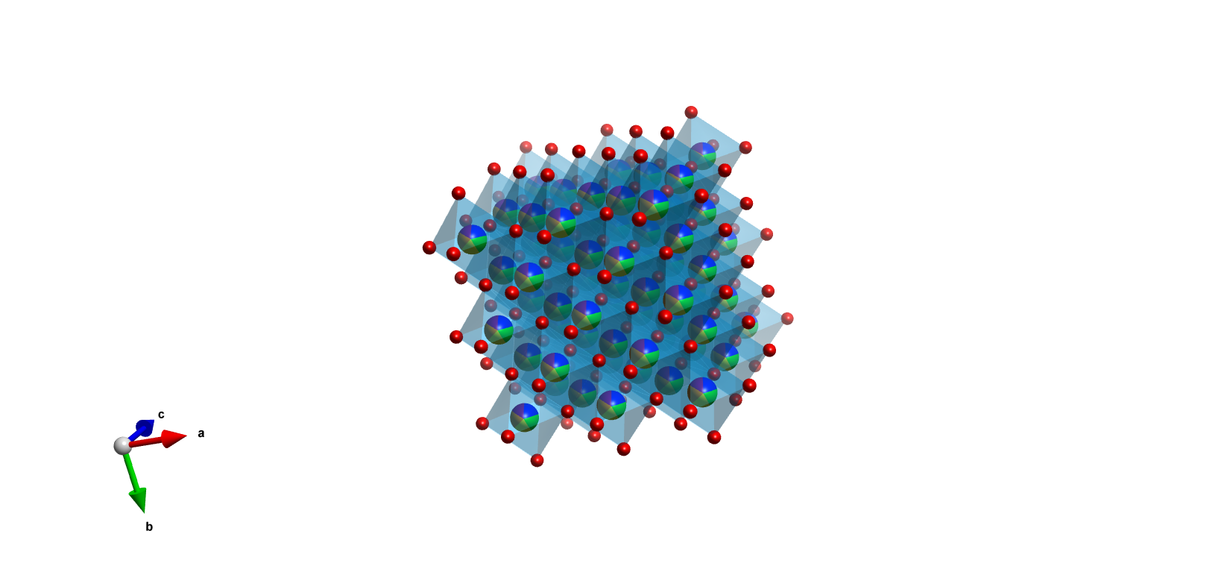
Investigating HEM structures, understanding the theoretical background, and developing new synthesis routes.
General Features
Application of HEMs for energy storage materials, electrolytes and utilization as catalysts.
Electrochemistry of HEM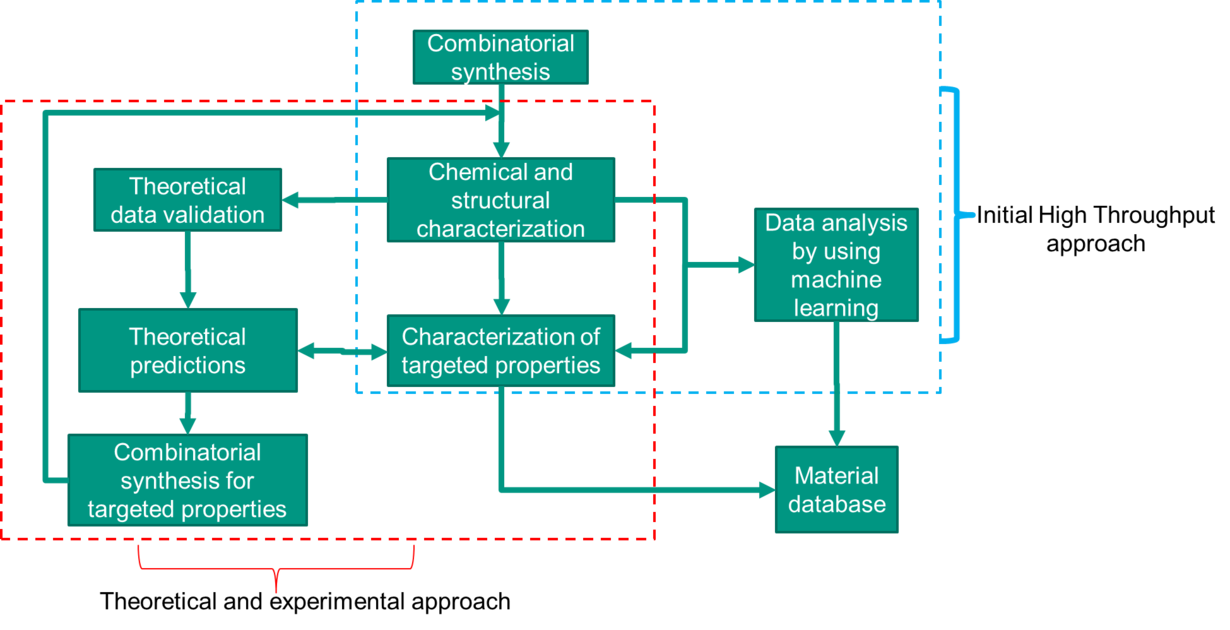
High-throuput methods for HEM using automated synthesis and analysis in combination with machine learning.
HTSA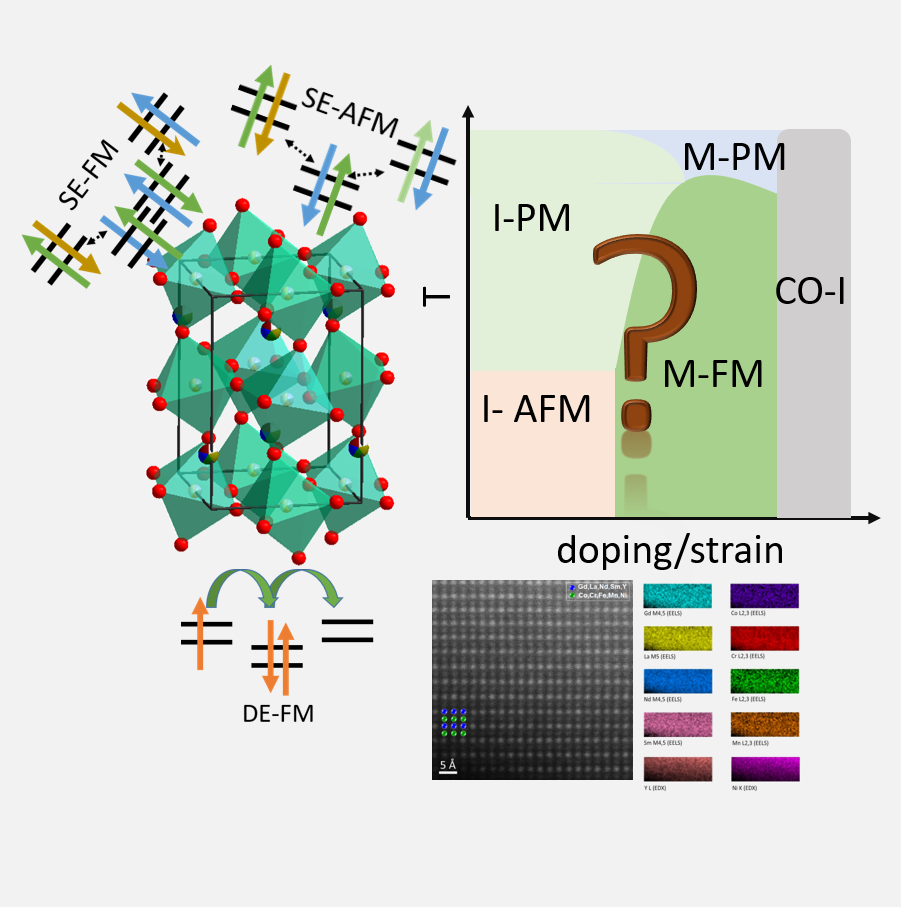
Exploring the magento-electronic phase space of HEMs utilizing chemical disorder and strain engineering
Mangeto-ElectronicsSelected Publications
Kante, M. V.; Lakshmi Nilayam, A. R.; Kreka, K.; Hahn, H.; Bhattacharya, S. S.; Velasco, L.; Tarancón, A.; Kübel, C.; Schweidler, S.; Botros, M.
2024. Journal of Physics: Energy, 6 (3), Art.-Nr.: 035001. doi:10.1088/2515-7655/ad423c
Kante, M. V.; Botros, M.; Schweidler, S.; Raj Lakshmi Nilayam, A.
2024, April 29. doi:10.35097/GcZKqFdyZsBbHMjv
Strauss, F.; Botros, M.; Breitung, B.; Brezesinski, T.
2024. Journal of Applied Physics, 135 (12), Art.-Nr.: 120901. doi:10.1063/5.0200031
Kante, M. V.; Nilayam, L. A. R. L.; Hahn, H.; Bhattacharya, S. S.; Elm, M. T.; Velasco, L.; Botros, M.
2024. Small, Art.-Nr.: 2309735. doi:10.1002/smll.202309735
Schweidler, S.; Brezesinski, T.; Breitung, B.
2024. Nature Energy, 9 (3), 240–241. doi:10.1038/s41560-024-01468-z
Triolo, C.; Schweidler, S.; Lin, L.; Pagot, G.; Di Noto, V.; Breitung, B.; Santangelo, S.
2023. Energy Advances, 2 (5), 667–678. doi:10.1039/D3YA00062A
Cadilha Marques, G.; Yang, L.; Liu, Y.; Wollersen, V.; Scherer, T.; Breitung, B.; Wegener, M.; Aghassi-Hagmann, J.
2023. Advanced Materials Technologies, 8 (22), Art.-Nr.: 2370121. doi:10.1002/admt.202370121
Schweidler, S.; Botros, M.; Strauss, F.; Wang, Q.; Ma, Y.; Velasco, L.; Cadilha Marques, G.; Sarkar, A.; Kübel, C.; Hahn, H.; Aghassi-Hagmann, J.; Brezesinski, T.; Breitung, B.
2024. Nature Reviews Materials. doi:10.1038/s41578-024-00654-5
Debastiani, R.; Kurpiers, C. M.; Lemma, E. D.; Breitung, B.; Bastmeyer, M.; Schwaiger, R.; Gumbsch, P.
2023. arxiv. doi:10.48550/arXiv.2312.16208
Schuetzke, J.; Schweidler, S.; Muenke, F. R.; Orth, A.; Khandelwal, A. D.; Breitung, B.; Aghassi-Hagmann, J.; Reischl, M.
2024. Advanced Intelligent Systems, 6 (3), Art.-Nr.: 2300501. doi:10.1002/aisy.202300501
Saghafi, M. K.; Vasantham, S. K.; Hussain, N.; Mathew, G.; Colombo, F.; Schamberger, B.; Pohl, E.; Marques, G. C.; Breitung, B.; Tanaka, M.; Bastmeyer, M.; Selhuber-Unkel, C.; Schepers, U.; Hirtz, M.; Aghassi-Hagmann, J.
2023. Advanced Functional Materials, 33 (51), Art.-Nr.: 2308613. doi:10.1002/adfm.202308613
He, Y.; Dreyer, S. L.; Ting, Y.-Y.; Ma, Y.; Hu, Y.; Goonetilleke, D.; Tang, Y.; Diemant, T.; Zhou, B.; Kowalski, P. M.; Fichtner, M.; Hahn, H.; Aghassi-Hagmann, J.; Brezesinski, T.; Breitung, B.; Ma, Y.
2024. Angewandte Chemie International Edition, 63 (7), e202315371. doi:10.1002/anie.202315371
Lee, S.; Bai, L.; Jeong, J.; Stenzel, D.; Schweidler, S.; Breitung, B.
2023. Electrochimica Acta, 463, 142775. doi:10.1016/j.electacta.2023.142775
Csík, D.; Baranová, G.; Džunda, R.; Zalka, D.; Breitung, B.; Hagarová, M.; Saksl, K.
2023. Coatings, 13 (7), 1219. doi:10.3390/coatings13071219
Cadilha Marques, G.; Yang, L.; Liu, Y.; Wollersen, V.; Scherer, T.; Breitung, B.; Wegener, M.; Aghassi-Hagmann, J.
2023. Advanced Materials Technologies, 8 (22), Art.-Nr.: 2300893. doi:10.1002/admt.202300893
Ma, Y.; Brezesinski, T.; Breitung, B.; Ma, Y.
2023. Matter, 6 (2), 313–315. doi:10.1016/j.matt.2023.01.008
Hu, H.; Scholz, A.; Dolle, C.; Zintler, A.; Quintilla, A.; Liu, Y.; Tang, Y.; Breitung, B.; Marques, G. C.; Eggeler, Y. M. M.; Aghassi-Hagmann, J.
2024. Advanced Functional Materials, 34 (20), Art.Nr.: 2302290. doi:10.1002/adfm.202302290
Lin, L.; Ding, Z.; Karkera, G.; Diemant, T.; Kante, M. V. V.; Agrawal, D.; Hahn, H.; Aghassi-Hagmann, J.; Fichtner, M.; Breitung, B.; Schweidler, S.
2023. Small Structures, 4 (9), Art.-Nr.: 2300012. doi:10.1002/sstr.202300012
Kumbhakar, M.; Khandelwal, A.; Jha, S. K.; Kante, M. V.; Keßler, P.; Lemmer, U.; Hahn, H.; Aghassi-Hagmann, J.; Colsmann, A.; Breitung, B.; Velasco, L.; Schweidler, S.
2023. Advanced Energy Materials, 13 (24), Art.-Nr.: 2204337. doi:10.1002/aenm.202204337
Lin, L.; Ding, Z.; Karkera, G.; Diemant, T.; Kante, M. V.; Agrawal, D.; Hahn, H.; Aghassi, J.; Fichtner, M.; Breitung, B.; Schweidler, S.
2023, May 16. doi:10.5445/IR/1000158543
Wang, K.; Hua, W.; Huang, X.; Stenzel, D.; Wang, J.; Ding, Z.; Cui, Y.; Wang, Q.; Ehrenberg, H.; Breitung, B.; Kübel, C.; Mu, X.
2023. Nature Communications, 14, Art.-Nr.: 1487. doi:10.1038/s41467-023-37034-6
Botros, M.; Janek, J.
2022. Science, 378 (6626), 1273–1274. doi:10.1126/science.adf3383
Wang, J.; Dreyer, S. L.; Wang, K.; Ding, Z.; Diemant, T.; Karkera, G.; Ma, Y.; Sarkar, A.; Zhou, B.; Gorbunov, M. V.; Omar, A.; Mikhailova, D.; Presser, V.; Fichtner, M.; Hahn, H.; Brezesinski, T.; Breitung, B.; Wang, Q.
2022. Materials Futures, 1 (3), Art.Nr. 035104. doi:10.1088/2752-5724/ac8ab9
Schweidler, S.; Tang, Y.; Lin, L.; Karkera, G.; Alsawaf, A.; Bernadet, L.; Breitung, B.; Hahn, H.; Fichtner, M.; Tarancón, A.; Botros, M.
2022. Frontiers in Energy Research, 10, Art.-Nr.: 983979. doi:10.3389/fenrg.2022.983979
Wang, K.; Hua, W.; Huang, X.; Stenzel, D.; Wang, J.; Ding, Z.; Cui, Y.; Wang, Q.; Ehrenberg, H.; Breitung, B.; Kübel, C.; Mu, X.
2023, January 11. doi:10.5445/IR/1000154295
Cui, Y.; Sukkurji, P. A.; Wang, K.; Azmi, R.; Nunn, A. M.; Hahn, H.; Breitung, B.; Ting, Y.-Y.; Kowalski, P. M.; Kaghazchi, P.; Wang, Q.; Schweidler, S.; Botros, M.
2022. Journal of Energy Chemistry, 72, 342–351. doi:10.1016/j.jechem.2022.05.032
Stenzel, D.; Zhou, B.; Okafor, C.; Kante, M. V.; Lin, L.; Melinte, G.; Bergfeldt, T.; Botros, M.; Hahn, H.; Breitung, B.; Schweidler, S.
2022. Frontiers in Energy Research, 10, Art.-Nr.: 942314. doi:10.3389/fenrg.2022.942314
Ma, Y.; Hu, Y.; Pramudya, Y.; Diemant, T.; Wang, Q.; Goonetilleke, D.; Tang, Y.; Zhou, B.; Hahn, H.; Wenzel, W.; Fichtner, M.; Ma, Y.; Breitung, B.; Brezesinski, T.
2022. Advanced Functional Materials, 32 (34), Art.Nr. 2202372. doi:10.1002/adfm.202202372
Schweidler, S.; Dreyer, S. L.; Breitung, B.; Brezesinski, T.
2022. Coatings, 12 (3), 402. doi:10.3390/coatings12030402
Wang, L.; Torkamanzadeh, M.; Majed, A.; Zhang, Y.; Wang, Q.; Breitung, B.; Feng, G.; Naguib, M.; Presser, V.
2022. Advanced sustainable systems, 6 (3), Artk.Nr:: 2100383. doi:10.1002/adsu.202100383
Lin, L.; Wang, K.; Sarkar, A.; Njel, C.; Karkera, G.; Wang, Q.; Azmi, R.; Fichtner, M.; Hahn, H.; Schweidler, S.; Breitung, B.
2022. Advanced Energy Materials, 12 (8), Art.-Nr. 2103090. doi:10.1002/aenm.202103090
Schweidler, S.; Dreyer, S. L.; Breitung, B.; Brezesinski, T.
2021. Scientific reports, 11 (1), Article no: 23381. doi:10.1038/s41598-021-02685-2
Wang, Q.; Velasco, L.; Breitung, B.; Presser, V.
2021. Advanced energy materials, 11 (47), Art. Nr.: 2102355. doi:10.1002/aenm.202102355
Ma, Y.; Ma, Y.; Dreyer, S. L.; Wang, Q.; Wang, K.; Goonetilleke, D.; Omar, A.; Mikhailova, D.; Hahn, H.; Breitung, B.; Brezesinski, T.
2021. Advanced Materials, 33 (34), Art. Nr.: 2101342. doi:10.1002/adma.202101342
Ma, Y.; Ma, Y.; Wang, Q.; Schweidler, S.; Botros, M.; Fu, T.; Hahn, H.; Brezesinski, T.; Breitung, B.
2021. Energy and Environmental Science, 14 (5), 2883–2905. doi:10.1039/d1ee00505g
Sukkurji, P. A.; Cui, Y.; Lee, S.; Wang, K.; Azmi, R.; Sarkar, A.; Indris, S.; Bhattacharya, S. S.; Kruk, R.; Hahn, H.; Wang, Q.; Botros, M.; Breitung, B.
2021. Journal of Materials Chemistry A, 9 (14), 8998–9009. doi:10.1039/d0ta10209a
Stenzel, D.; Issac, I.; Wang, K.; Azmi, R.; Singh, R.; Jeong, J.; Najib, S.; Bhattacharya, S. S.; Hahn, H.; Brezesinski, T.; Schweidler, S.; Breitung, B.
2021. Inorganic chemistry, 60 (1), 115–123. doi:10.1021/acs.inorgchem.0c02501
Jeong, J.; Singaraju, S. A.; Aghassi-Hagmann, J.; Hahn, H.; Breitung, B.
2020. ChemElectroChem, 7 (13), 2735–2739. doi:10.1002/celc.202000305
Wang, J.; Stenzel, D.; Azmi, R.; Najib, S.; Wang, K.; Jeong, J.; Sarkar, A.; Wang, Q.; Sukkurji, P. A.; Bergfeldt, T.; Botros, M.; Maibach, J.; Hahn, H.; Brezesinski, T.; Breitung, B.
2020. Electrochem, 1 (1), 60–74. doi:10.3390/electrochem1010007
Wang, J.; Cui, Y.; Wang, Q.; Wang, K.; Huang, X.; Stenzel, D.; Sarkar, A.; Azmi, R.; Bergfeldt, T.; Bhattacharya, S. S.; Kruk, R.; Hahn, H.; Schweidler, S.; Brezesinski, T.; Breitung, B.
2020. Scientific reports, 10, Art.-Nr.: 18430. doi:10.1038/s41598-020-75134-1
Lin, L.; Wang, K.; Azmi, R.; Wang, J.; Sarkar, A.; Botros, M.; Najib, S.; Cui, Y.; Stenzel, D.; Anitha Sukkurji, P.; Wang, Q.; Hahn, H.; Schweidler, S.; Breitung, B.
2020. Journal of materials science, 55, 16879–16889. doi:10.1007/s10853-020-05183-4
Sarkar, A.; Breitung, B.; Hahn, H.
2020. Scripta materialia, 187, 43–48. doi:10.1016/j.scriptamat.2020.05.019
Breitung, B.; Wang, Q.; Schiele, A.; Tripković, Đ.; Sarkar, A.; Velasco, L.; Wang, D.; Bhattacharya, S. S.; Hahn, H.; Brezesinski, T.
2020. Batteries & supercaps, 3 (4), 361–369. doi:10.1002/batt.202000010
Chellali, M. R.; Sarkar, A.; Nandam, S. H.; Bhattacharya, S. S.; Breitung, B.; Hahn, H.; Velasco, L.
2019. Scripta materialia, 166, 58–63. doi:10.1016/j.scriptamat.2019.02.039
Sarkar, A.; Wang, Q.; Schiele, A.; Chellali, M. R.; Bhattacharya, S. S.; Wang, D.; Brezesinski, T.; Hahn, H.; Velasco, L.; Breitung, B.
2019. Advanced materials, 1806236. doi:10.1002/adma.201806236
Wang, Q.; Sarkar, A.; Li, Z.; Lu, Y.; Velasco, L.; Bhattacharya, S. S.; Brezesinski, T.; Hahn, H.; Breitung, B.
2019. Electrochemistry communications, 100, 121–125. doi:10.1016/j.elecom.2019.02.001
Sarkar, A.; Velasco, L.; Wang, D.; Wang, Q.; Talasila, G.; Biasi, L. de; Kübel, C.; Brezesinski, T.; Bhattacharya, S. S.; Hahn, H.; Breitung, B.
2018. Nature Communications, 9 (1), Article number: 3400. doi:10.1038/s41467-018-05774-5





_rdax_200x296s.jpg)
_rdax_200x296s.jpg)



