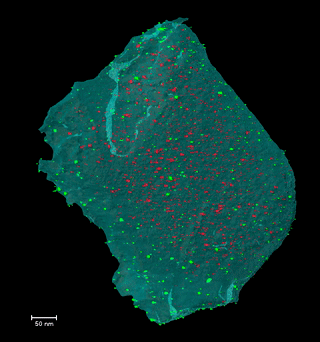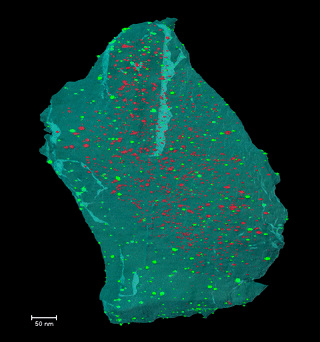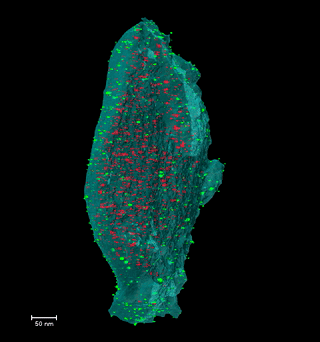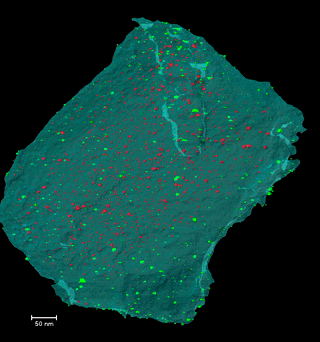
Disclosing the leaching behaviour of Pd@CMK3 catalysts in formic acid decomposition by electron tomography
Understanding the leaching behaviour of Pd@CMK3 catalysts during formic acid decomposition requires examining how pore confinement, nanoparticle mobility, and reactor hydrodynamics co-govern catalyst stability and performance. The article demonstrates that leaching is not a singular degradation mechanism but arises from a dynamic balance between Pd dissolution, particle sintering, and redeposition processes that unfold differently depending on the reaction environment and the accessibility of mesoporous channels within the CMK3 support. In batch reactors, where reactants and catalyst coexist in a closed volume, dissolved Pd species encounter restricted diffusion paths and prolonged residence times, conditions that encourage redeposition onto the internal and external surfaces of the mesoporous carbon. This enhances the likelihood of retention of active metal, even as nanoparticle size distributions evolve through ripening and aggregation, illustrating how confinement within sub-nanometer to nanometer pores can partially mitigate metal loss by physically and kinetically stabilizing ultrafine particles. By contrast, continuous-flow reactors promote pronounced leaching through persistent solvent flux, which continuously extracts dissolved Pd from the porous matrix and limits redeposition, leading to severe depletion of internal particles and preferential redeposition only near external surfaces, where fluid transport is more accessible. This interplay underscores that catalyst architecture—specifically the spatial distribution of ultrafine nanoparticles relative to internal versus external pore networks—must be engineered in concert with reactor design, as identical formulations can display markedly different degradation pathways depending on operational mode. Moreover, electron tomography is a indispensable three-dimensional analytical technique capable of resolving the nuanced redistribution of nanoparticles at nanometric scale, revealing structural evolutions that conventional 2D characterization obscures and establishing reliable correlations between nanoscale structural change and catalytic durability. Collectively, these insights emphasize that controlling metal leaching in heterogeneous catalysts is not solely a function of chemical composition, but also a consequence of physical confinement, fluid dynamics, and surface accessibility—all critical parameters for designing next-generation catalysts optimized for hydrogen release from formic acid and other liquid organic hydrogen carriers.

Details and further work are published at:
- Huang, X.; Barlocco, I.; Villa, A.; Kübel, C.; Wang, D. Disclosing the Leaching Behaviour of Pd@CMK3 Catalysts in Formic Acid Decomposition by Electron Tomography. Nanoscale Advances, 2023, 5, 1141–1151. DOI: 10.1039/d2na00664b.
Alloyed Supported Catalysts in Formic Acid Decomposition
The catalytic improvement observed in Pd–Au alloyed systems reflects a sophisticated interplay between nanoscale structure, surface chemistry, and reaction energetics, which collectively determine how efficiently formic acid can be converted into hydrogen under mild conditions. The introduction of gold into palladium matrices does more than merely dilute active sites; it fundamentally modifies Pd’s electronic environment through electron transfer processes that alter adsorption strengths and reaction pathways. This modification is evident in the binding energy shifts and surface composition changes revealed through XPS, where Au donates electron density to Pd and facilitates the preferential exposure of palladium atoms at the nanoparticle surface. Such electronic restructuring suppresses CO poisoning by weakening the adsorption of poisoning intermediates relative to dehydrogenation species, a critical limitation in monometallic Pd catalysts. At the same time, STEM and EDX analyses confirm that alloying yields homogeneous and stable Pd–Au distributions, supporting the premise that compositional tuning at the nanoscale is central to optimizing catalytic behavior. Complementary DFT calculations illuminate these experimental observations with atomistic insight: bimetallic Pd₉Au₆ clusters not only bind formic acid exothermically but also steer decomposition along the more desirable dehydrogenation route, producing CO₂ and H₂ while energetically disfavoring the dehydration pathway. This preference emerges from a delicate balance of ligand and strain effects, which reduce ensemble arrangements prone to CO formation and stabilize reaction intermediates. Furthermore, the stronger adhesion between bimetallic nanoparticles and carbon nanofiber supports—compared to monometallic analogues—confers resistance to sintering and agglomeration, ensuring sustained catalytic performance over multiple reaction cycles without significant loss of activity or selectivity. Collectively, these findings position Pd–Au alloyed catalysts as strategically engineered systems in which nanoscale alloying, support interactions, and electronic tuning converge to elevate hydrogen production efficiency, demonstrating how rational design principles can transform molecular-scale mechanisms into tangible advancements for sustainable energy applications.

Details and further work are published at:
- Barlocco, I.; Capelli, S.; Lu, X.; Bellomi, S.; Huang, X.; Wang, D.; Prati, L.; Dimitratos, N.; Roldan, A.; Villa, A. Disclosing the Role of Gold on Palladium–Gold Alloyed Supported Catalysts in Formic Acid Decomposition. ChemCatChem, 2021, 13, 4210–4222. https://doi.org/10.1002/cctc.202100886
Highly Active Oxidation Catalysts
In advanced heterogeneous catalysis, several interrelated concepts define the performance and stability of noble-metal systems. The notion of noble-metal dispersion describes how Pd exists either as atomically dispersed species or as nanoclusters whose nuclearity dictates reactivity; ceria–metal interactions refer to the strong anchoring of Pd on CeO₂, which stabilizes oxidized species yet can also drive redispersion under oxidizing conditions; the cluster formation threshold reflects the local surface noble-metal concentration required for transition from isolated atoms to catalytically competent PdOₓ clusters; and spatial confinement on nano-islands—here realized by embedding CeO₂ domains within an Al₂O₃ matrix—limits Pd mobility, mitigating sintering and over-dispersion, while enabling in situ cluster formation even at low overall loadings (0.5 wt%).

Details and further work are published at:
- Gashnikova, D., Maurer, F., Sauter, E., Bernart, S., Jelic, J., Dolcet, P., Maliakkal, C. B., Wang, Y., Wöll, C., Studt, F., Kübel, C., Casapu, M., & Grunwaldt, J.-D. (2024). Highly Active Oxidation Catalysts through Confining Pd Clusters on CeO₂ Nano-Islands. Angewandte Chemie International Edition, 63(35), e202408511. https://doi.org/10.1002/anie.202408511