Hydrogen Storage System
Among various storage strategies, metal hydrides offer high volumetric hydrogen density and favorable reversibility, making them promising candidates for solid-state hydrogen storage. One of their main benefits is high stability, as they operate safely under low working pressures, typically below 20 bar, which reduces the risk of leakage or explosion compared to compressed gas storage. Additionally, these systems provide excellent space efficiency due to their high volumetric hydrogen density, allowing more hydrogen to be stored in a smaller volume. Another key advantage is their suitability for long-term or semi-permanent storage, since the absorbed hydrogen remains securely bound within the metal lattice until released under controlled conditions.
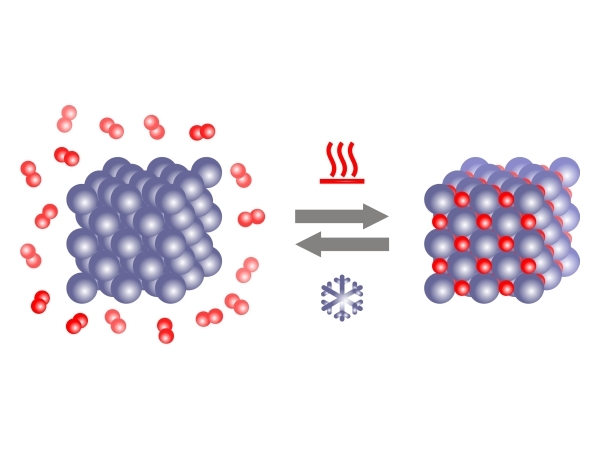
Figure 1. Schematic representation of hydrogenation and dehydrogenation [1].
However, their performance is strongly governed by atomic-scale processes such as hydrogen diffusion, defect evolution, and phase transformation, which are still not fully understood. Alloys like TiFe and Mg-based hydrides exhibit high capacity and reversibility but often suffer from kinetic limitations, capacity degradation, and structural instability during hydrogen cycling. To develop more efficient hydrogen-storage materials, it is essential to uncover how atomic structure, strain, and chemical bonding evolve as hydrogen is absorbed and released.
Recent advances in transmission electron microscopy, particularly four-dimensional scanning transmission electron microscopy (4D-STEM) and in-situ gas TEM, now allow direct visualization of hydrogen–metal interactions with nanometer and even atomic resolution. These techniques provide spatially resolved maps of strain, atomic density, and local structure that reveal how hydrogen incorporation alters the lattice and how defects or interfaces affect diffusion pathways. When combined with data-driven analysis methods such as machine-learning-assisted pattern recognition and statistical decomposition of diffraction data, these approaches enable quantitative reconstruction of local environments and phase evolution within hydrides. Through correlative analysis of structural, mechanical, and chemical information, it becomes possible to track how hydrogen-induced strain fields nucleate, propagate, and relax during absorption and desorption.
This microscopy-based understanding provides an experimental foundation for linking atomic configurations to macroscopic storage behavior. It clarifies how nanoscale heterogeneity and structural disorder influence hydrogen kinetics, stability, and reversibility. Moreover, by visualizing phase boundaries, defect networks, and strain localization, electron microscopy helps to identify microstructural design strategies that enhance cycling performance—such as introducing catalytic interfaces, tailoring grain boundaries, or stabilizing intermediate hydride phases. Ultimately, this research demonstrates how the integration of advanced electron microscopy, in-situ experimentation, and data-driven analysis can reveal the dynamic behavior of hydrogen in solids, paving the way for the rational design of next-generation hydrogen-storage materials with improved efficiency, durability, and tunable functionality.
Details and further work are published at:
- Fraunhofer Institute for Manufacturing Technology and Advanced Materials (IFAM). Materials Processing & Analysis. Available at: https://www.ifam.fraunhofer.de/en/Aboutus/Locations/Dresden/HydrogenTechnology/hydrides/materials-processing---analysis.html
- U.S. Department of Energy, Materials-based hydrogen storage, Energy.gov, n.d. Available at: https://www.energy.gov/eere/fuelcells/materials-based-hydrogen-storage

